Abstract
Treatment of acute hepatitis C virus (HCV) infection leads to a sustained virologic response (SVR) in the vast majority of patients, although the clinical predictors of these favorable responses are not well understood. In chronic infection, the most potent predictor of a SVR is complete viral suppression after four weeks of treatment, also known as a rapid virologic response (RVR). However, few patients with genotype-1 infection and high-level viremia ever achieve this benchmark. In two separate cohorts of patients with acute HCV infection, we demonstrate that rapid virologic clearance and low-level viremia (<400,000 IU/ml) are highly prevalent, regardless of genotype.
Keywords: Acute hepatitis C infection, treatment, pegylated interferon, correctional medicine, prison, rapid virologic response
INTRODUCTION
In acute hepatitis C virus (HCV) infection, overall sustained virologic response (SVR) rates are much higher than those seen in chronic infection, although the clinical predictors of these favorable outcomes are not well understood. In patients with chronic infection, the recent identification of rapid virologic clearance (defined as undetectable hepatitis C viremia after four weeks of therapy), has been lauded as the single most important factor in determining treatment outcomes [1, 2]. The ability to achieve rapid virologic clearance is governed by two primary factors: the infecting genotype and the level of the patient’s viremia prior to treatment [3, 4].
RVR is attained by approximately two-thirds of patients with genotypes 2 and 3 infection treated with pegylated interferon plus ribavirin [5]. In contrast, this benchmark is achieved in only the minority of patients with genotype 1 infection (16 to 23 percent) [1]. These data support the clinical observation that patients with genotype 2 or 3 infection have significantly higher overall sustained virologic response rates than genotype 1-infected patients. In addition to genotype, lower levels of viremia are also strongly correlated with virologic suppression. However, only a minority of patients with chronic HCV infection have low levels of viremia (i.e., < 400,000 IU/ml) at baseline [4, 5].
In patients with acute HCV infection, virologic responses after four weeks of treatment have not been well-defined. Our goal was to assess the prevalence of RVR responses to pegylated interferon and ribavirin therapy among patients who were diagnosed with acute HCV infection within two separate cohorts. We demonstrate that: 1) rapid virologic clearance is common during treatment for acute HCV infection, regardless of genotype and 2) rapid virologic clearance may be related to the high prevalence of low level viremia in newly acquired infection.
METHODS
We assessed four-week HCV RNA responses among patients who were treated for acute HCV infection within two separate cohorts. The first cohort was identified prospectively beginning in October 2006 through the Massachusetts Department of Corrections. Upon admission, newly incarcerated inmates were screened for a history of recent onset injection drug use (IDU) and prior HCV testing. During the initial physical examination at two correctional intake sites, brief interviews of 3,248 inmates were conducted by healthcare providers from the University of Massachusetts Healthcare Services. Of these inmates, 141 “high-risk” individuals without a history of HCV infection were further screened by history and laboratory evaluation (e.g., symptoms/signs of acute hepatitis, HCV seroconversion, elevations of aminotransferases >7x ULN, and presence of HCV RNA). After diagnosis of acute HCV infection, we evaluated HCV RNA at baseline, four and 10 weeks and offered therapy only to those with persistent viremia based on our previously established screening protocol [6]. Full details of our diagnostic approach are described elsewhere [McGovern, et al in submission].
Patients with persistent viremia exceeding 10 weeks from diagnosis were offered 24 weeks of treatment with pegylated interferon alfa2b and ribavirin (800 mg daily for genotypes 2 or 3; weight-based ribavirin for genotype 1 infected patients at 13 mg/kg). Patients with low-level viremia (i.e., HCV RNA level <10,000 IU/ml) at week 10 underwent additional testing at week 14 to determine possible late clearance before initiation of treatment. Those who were administered combination therapy for persistent viremia had virologic monitoring (VERSANT HCV RNA 2.0, Bayer Diagnostics) on a monthly basis until clearance was documented. HCV RNA was also assessed at the end of treatment and at the six-month after discontinuation of therapy. HCV genotype was determined by VERSANT-LiPA HCV 2.0 (Bayer, Tarrytown, NY).
In the second cohort, we retrospectively reviewed 68 records from community patients diagnosed with acute HCV infection at a tertiary care center (Massachusetts General Hospital) from 1997 to 2007. Serial HCV RNA levels were obtained (mean 5.4 samples/patient); the timing and frequency of the samples were at the discretion of the two practitioners who provided care for these patients. The mean duration of sampling prior to treatment was 17.6 weeks. From the overall cohort, 27 were treated for persistent viremia (6 patients were initially described in a previous report [7]. We selected ten patients who had HCV RNA testing (Cobas Amplicor HCV Monitor) after four weeks of pegylated interferon and ribavirin for inclusion in this report.
Ethical issues
All acute HCV-infected study subjects gave written informed consent for observational studies of the virology, immunology and clinical course of infection. These protocols conform to the 1975 Helsinki guidelines for the conduct of human research and were approved by each hospital’s Institutional Review Board. For incarcerated subjects, the Lemuel Shattuck Hospital Human Research Review Committee includes a prisoner advocate.
RESULTS
Identification of patients with acute HCV infection and virologic outcomes
We identified 25 patients with acute HCV infection and persistent viremia who underwent combination therapy with pegylated interferon and ribavirin and had week 4 viral load testing: 15 patients identified prospectively from the prison-based cohort and 10 identified retrospectively from the community-based cohort (17 males; mean age: 31) (see table 1). The racial distribution was as follows: 22 Caucasian; 2 Hispanic and 1 person who was half Caucasian and half African-American; Risk factors for HCV acquisition included IDU (n=20), sexual transmission (n=4) and unknown route (n=1). Two patients were known to be HIV-seropositive in the community cohort. No new diagnoses of HIV infection were made.
Table 1.
Demographics and treatment outcomes
| ID # | Age | Gender | Ethnicity | Genotype | Pre-treatment HCV RNA (IU/ml) |
Treatment Weeks | RVR | SVR |
|---|---|---|---|---|---|---|---|---|
| Incarcerated Cohort | ||||||||
| 1 | 21 | M | Caucasian | 1a | 363,000 | 22 | yes | yes |
| 2 | 31 | M | Caucasian | 1a | 22,200 | 24 | yes | yes |
| 3 | 29 | M | Caucasian | 1a | 93,699 | 25 | yes | yes |
| 4 | 25 | F | Caucasian | 2b | 1,665,560 | 15 | yes | yes |
| 5 | 34 | M | Caucasian | 1a | 252,532 | 24 | yes | yes |
| 6 | 41 | M | Caucasian | 4a | 88,574 | 24 | yes | yes |
| 7 | 29 | M | Caucasian | 3a | 834,400 | 26 | yes | yes |
| 8 | 35 | M | Caucasian/Hispanic | 1a | 32,366 | 16 | yes | yes |
| 9 | 24 | M | Caucasian | 1a | 261,437 | 24 | yes | yes |
| 10 | 19 | F | Caucasian | 1a | 131,819 | 24 | yes | viral breakthrough |
| 11 | 32 | M | Caucasian | 1a | 546,478 | 20 | no | viral breakthrough |
| 12 | 30 | M | Caucasian | 4acd | 1,590 | 16 | yes | lost to follow up |
| 13 | 24 | F | Caucasian | 3a | 190,778 | 12 | yes | lost to follow up |
| 14 | 39 | M | Caucasian | 3a | 93,185 | 12 | yes | yes |
| 15 | 40 | M | Caucasian | 1a | 1,790,000 | 28 | yes | no |
| Community Cohort | ||||||||
| 16 | 63 | F | Caucasian | 1 | 95,800 | 12 | yes | yes |
| 17 | 36 | M | Caucasian | 1b | 1,230 | 24 | yes | yes |
| 18 | 20 | F | Caucasian | 1a | 235,000 | 24 | yes | yes |
| 19 | 26 | F | Caucasian | 1a | 648 | 24 | yes | yes |
| 20 | 20 | M | Caucasian/Black | 1ab | > 700000 | 48 | no | yes |
| 21 | 27 | M | Caucasian | 1 | 292,000 | 24 | yes | lost to follow up |
| 22 | 29 | M | Caucasian | 1 | > 700000 | 48 | yes | yes |
| 23 | 20 | F | Caucasian | 1 | > 700000 | 24 | yes | yes |
| 24 | 40 | F | Caucasian | 3a | > 700000 | 12 | yes | yes |
| 25 | 41 | M | Hispanic | 3a | 1,385 | 24 | yes | yes |
HCV RNA monitoring of patient 1 utilized bDNA Quantiplex, Chiron Corporation for all time points.
A course of 48 weeks of therapy was recommended to patient 15 who delayed treatment for 27 months due to depression; he self-discontinued treatment after 27 weeks with subsequent virologic rebound.
Patient 22 and 25 were HIV seropositive.
Nineteen patients had genotype 1 or 4 infection (17 and 2 patients, respectively), while 6 patients had genotype 2 or 3 infection (1 and 5 patients, respectively). The mean HCV RNA level at enrollment was 329,210 IU/ml (range: 1,004 – 1,745,260 IU/ml) while the mean HCV RNA prior to treatment had increased to 391,747 IU/ml (range: 1,385 – 1,665,560 IU/ml), although this trend was not statistically significant. Pretreatment low-level viremia was documented in 17 of 25 patients (68%), including 12 of 17 (70.6 percent) with genotype 1 infection.
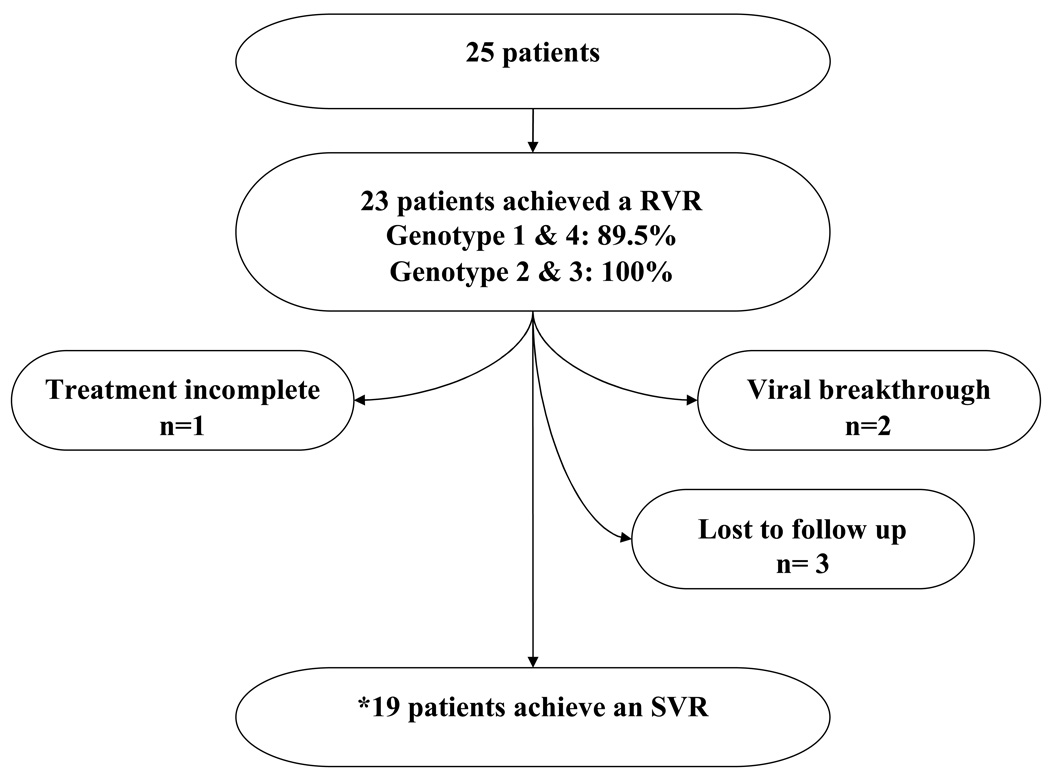
Rapid virologic clearance was achieved in 23 of 25 patients overall including two patients with a history of HIV infection. RVR rates for patients infected with genotypes 1 and 4 were 89.5 percent (17 of 19 patients) and were 100 percent in patients with genotypes 2 and 3 infection (6 of 6 patients). Assuming treatment failure in those patients who were lost to follow up (n=3), the overall response rate was 72 percent (18 of 25) (see figure 1).
Figure 1.
Treatment outcomes in combined cohort
*Eighteen out of 19 who attained a RVR achieved a SVR.
The patients were treated for a mean duration of 22.8 weeks (range, 12 to 48 weeks); one patient was offered 48 weeks of therapy due to delayed therapeutic intervention secondary to uncontrolled depression. Adverse events were typical of combination therapy and included severe fatigue (n=5), headache (n=4), loss of appetite (n=2), and new onset hypothyroidism (n=1); one patient received one dose of erythropoietin due to anemia; no patients required growth factors for neutropenia. Virologic breakthrough was documented in patients 10 and 11, both of whom had genotype 1 infection. Patient 10 admitted to poor ribavirin adherence towards the last two months of therapy. Although patient 11 was adherent, other factors may have contributed to his suboptimal response. These factors included a dramatic viral load increase between enrollment and pretreatment (22,635 to 546,478 IU/ml) as well as a marked increase in his baseline obesity. A fasting insulin level and a glucose concentration were normal at the time of treatment discontinuation.
DISCUSSION
Treatment for acute HCV infection led to rapid virologic suppression in nearly all patients, regardless of genotype. Of the 17 patients with genotype 1 infection, 88.2% achieved rapid virologic clearance. This observation is in marked contrast to the low RVR rates of approximately 16 to 23 percent seen in chronic HCV genotype-1 infection [1].
Studies in patients with chronic HCV infection have demonstrated that low level viremia (<400,000 IU/mL) and RVR are strongly linked. However, low-level viremia is distinctly uncommon in the most difficult-to-treat genotypes. For example, in one retrospective study of 1550 HCV genotype 1-infected patients who were treated with pegylated interferon alfa-2a/ribavirin, only 13 percent met this criterion [4]. Low-level viremia, which is distinctly uncommon in HIV/HCV coinfected patients, also informs RVR and ultimate clinical outcomes in this difficult-to-treat patient population [8].
In contrast to chronic disease, antiviral therapy leads to a sustained virologic response in the vast majority of patients with acute HCV infection [9]. The much higher RVR rates that are seen in acute HCV infection may be linked to prevalent low-level viremia as demonstrated in our cohort of patients with acute infection. The only patient in the incarcerated cohort who did not attain a RVR had more than a log-fold increase in viremia (>500,000 IU/ml) prior to treatment; one may hypothesize that he may have had a better outcome with earlier initiation of therapy at the time of lower viremia. The change in viral set-point to higher levels may signal a transition from acute to chronic HCV infection with onset of T-cell immune dysfunction [10]. In this setting, treatment with an immunomodulatory agent (e.g. interferon) may be less effective, particularly in genotype-1 infected patients. In contrast, the level of viremia may not be as important in patients with more interferon-responsive genotypes (ie, HCV genotypes 2 or 3).
Whether low-level viremia in acute infection is reflective of immunologic containment is unknown; patients with acute infection may have some relevant cellular responses, even if viremia persists [10, 11]. During acute infection, when acquired immunity is most likely to be detected, early treatment appears to facilitate a favorable balance between host-virus dynamics. In contrast, low level viremia may be distinctly uncommon in chronic infection due to the loss of immunologic control as suggested by in vitro data [12, 13]. Virally-induced signaling pathways (through interferon regulatory factor 3) that are favorable to the host may be abolished over time through inactivation of interferon-responsive genes (such as RIG-1) [12]. Moreover, the serine protease of HCV has recently been found to cleave signaling molecules essential to innate immunity [13]; the exact in vivo timing of activation of viral evasion mechanisms is currently under investigation. Thus, acute phase HCV may represent an immunologically favorable state before the virus has escaped from both innate and acquired immunity. Acute phase HCV is also associated with less diversity and complexity of an individual’s HCV quasispecies, which in the chronic phase has also been associated with favorable treatment outcomes [14]. The contribution of the immune system may be particularly important when administering the currently available antiviral therapies, which target upregulation of general antiviral genes, rather than specific HCV viral proteins [15].
Limitations of the current study include the small number of patients with acute HCV infection; thus, our observations should be confirmed by others. One strength of our study is the large representation of injection drug users who are often underrepresented in treatment studies of HCV infection. Furthermore, we were able to demonstrate the feasibility of treating acute HCV infection within the correctional setting.
In summary, in patients with acute HCV infection, rapid virologic clearance rates are commonly observed and are closely associated with SVR, regardless of genotype. High rates of RVR appear to be closely linked to low levels of viremia, which are more common in acute HCV infection. This virologic parameter may be particularly important in genotype 1-infected patients who have generally lower rates of SVR in response to treatment.
Acknowledgement
We especially thank the individuals who consented to take part in this study. We acknowledge Arthur Brewer and Warren Ferguson of University of Massachusetts Medical School Correctional Health and the providers at MCI-Framingham and MCI-Concord for their support. Funding sources: National Institutes of Health / National Institute of Allergy and Infectious Diseases (Hepatitis C Cooperative Center U19 AI066345, K23 AI054379 to AYK, Harvard University Center for AIDS Research P30 AI060354).
Footnotes
These data were presented during an oral presentation on November 1, 2008 at the 59TH Conference of the American Association for the Study of Liver Diseases in San Francisco, CA.
Conflict of Interest Statement: AYK, CEB, EHN, LLR, ACB, and MJB report no conflicts of interest. BHM is on the speaker’s bureau of Roche Pharmaceuticals. RTC receives research grant support from Roche Labs and Schering-Plough.
REFERENCES
- 1.Jensen DM, Morgan TR, Marcellin P, et al. Early identification of HCV genotype 1 patients responding to 24 weeks peginterferon alpha-2a (40 kd)/ribavirin therapy. Hepatology. 2006;43:954–960. doi: 10.1002/hep.21159. [DOI] [PubMed] [Google Scholar]
- 2.Ferenci P, Fried MW, Shiffman ML, et al. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40 KD)/ribavirin. J Hepatol. 2005;43:425–433. doi: 10.1016/j.jhep.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Torres M, Sulkowski M, Chung RT, Hamzeh FM, Jensen DM. Association of pretreatment and on-treatment factors with rapid virologic response in HCV genotype 1-infected patients treated with peginterferon alfa-2a/ribavirin. Boston, MA: American Association for the Study of Liver Diseases; 2007. Nov 2–6, Abstract #1305. [Google Scholar]
- 5.Shiffman ML, Suter F, Bacon BR, et al. Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Eng J M. 2007;357:124–134. doi: 10.1056/NEJMoa066403. [DOI] [PubMed] [Google Scholar]
- 6.McGovern B, Wurcel A, Kim AY, et al. Acute hepatitis C virus infection in incarcerated injection drug users. Clin Infect Dis. 2006;42:1663–1670. doi: 10.1086/504327. [DOI] [PubMed] [Google Scholar]
- 7.Corey KE, Ross AS, Wurcel A, et al. Outcomes and treatment of acute hepatitis C virus infection in a United States population. Clinical Gastroenterology and Hepatology. 2006;4:1278–1282. doi: 10.1016/j.cgh.2006.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin-Carbonero L, Nuñez M, Mariño A, et al. Undetectable hepatitis C virus RNA at week 4 as predictor of sustained virological response in HIV patients with chronic hepatitis C. AIDS. 2008;22:15–21. doi: 10.1097/QAD.0b013e3282f1da99. [DOI] [PubMed] [Google Scholar]
- 9.Maheshwari A, Ray S, Thuluvath PJ. Acute hepatitis C. Lancet. 2008;372:321–332. doi: 10.1016/S0140-6736(08)61116-2. [DOI] [PubMed] [Google Scholar]
- 10.Cox AL, Mosbruger T, Lauer GM, Pardoll D, Thomas DL, Ray SC. Comprehensive analyses of CD8+ T cell responses during longitudinal study of acute human hepatitis C. Hepatology. 2005;42:104–112. doi: 10.1002/hep.20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahman F, Heller T, Sobao Y, et al. Effects of antiviral therapy on the cellular immune response in acute hepatitis C. Hepatology. 2004;40:87–97. doi: 10.1002/hep.20253. [DOI] [PubMed] [Google Scholar]
- 12.Sumpter R, Loo Y, Foy E, et al. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol. 2005;79:2689–2699. doi: 10.1128/JVI.79.5.2689-2699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meylan E, Curran J, Hofmann K, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 14.Shire NJ, P.S. H, Rouster SD, et al. HCV kinetics, quasispecies, and clearance in treated HCV-infected and HCV/HIV-1-coinfected patients with hemophilia. Hepatology. 2006;44:1146–1157. doi: 10.1002/hep.21374. [DOI] [PubMed] [Google Scholar]
- 15.McGovern BH, Abu Dayyeh BK, Chung RT. Avoiding therapeutic pitfalls: the rational use of specifically targeted agents against hepatitis C infection. Hepatology. 2008;48:1700–1712. doi: 10.1002/hep.22563. [DOI] [PubMed] [Google Scholar]