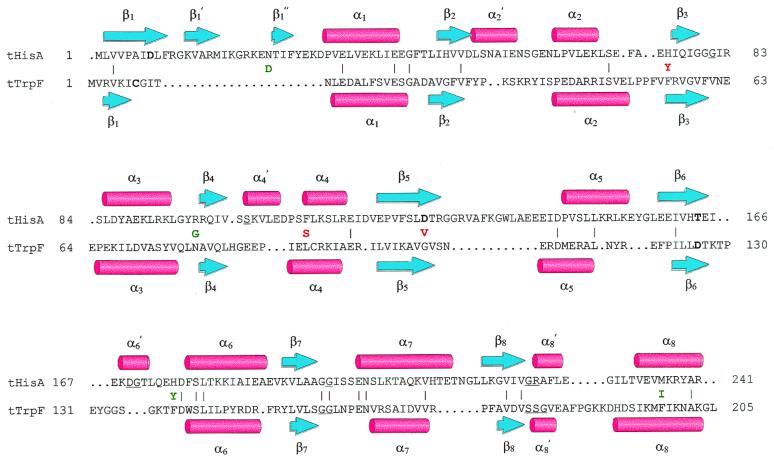
Figure 4.
Structure-based amino acid sequence alignment of tHisA and tTrpF. The backbone atoms from the x-ray structures of tHisA (8) and tTrpF (23) were superimposed with an rms deviation of 2.2 Å (24). β-Strands are represented by blue arrows, α-helices by red cylinders. Identical residues (10%) between tHisA and tTrpF are indicated by (|). Catalytically important residues of wild-type tHisA (Asp-8, Asp-127, and Thr-164; S. Schmidt, M. Henn-Sax, and R.S., unpublished data) and of wild-type tTrpF (Cys-7 and Asp-126; ref. 25) are in boldface. Residues involved in binding of the single phosphate moiety of PRA and of the two phosphate moieties of ProFAR are underlined. For tHisA, these residues were identified by a structure-based sequence alignment with the related tHisF protein, whose x-ray structure showed two phosphate ions bound to the active site (8). The acquired amino acids of tHisA_1 (green) and tHisA_2 (red) are given between the tHisA and the tTrpF sequences.