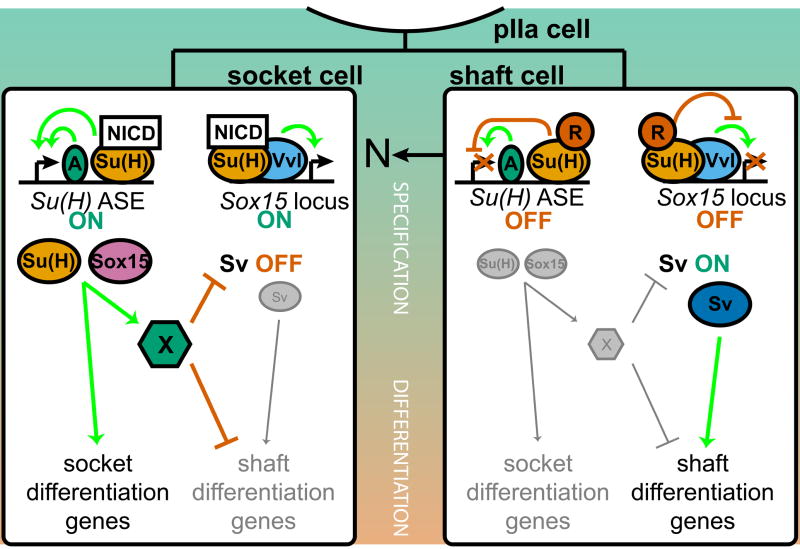
Fig. 7.
Summary model of the collaborative roles of Su(H) and Sox15 as N pathway targets required for socket cell differentiation. In the N signal-resistant shaft cell (right), Su(H) in its default repression mode keeps both the Su(H) ASE and the Sox15 socket enhancer off (in the latter case, despite the presence of Vvl). The result is that the Pax family factor Sv accumulates to high levels in the shaft cell and directs its differentiation program. Successful activation of the N pathway in the socket cell (left) relieves Su(H)-mediated repression of the two enhancers in this cell. The initially low basal level of Su(H), now complexed with NICD and Mam, contributes critically to the rapid (auto)activation of the Su(H) ASE, but acts permissively on the Sox15 enhancer, allowing Vvl and other factors to activate Sox15 gradually. Su(H) and Sox15 then function collaboratively (not hierarchically) to activate target genes required primarily for the later (physiological) phase of socket cell differentiation. They also collaborate to shut off continued transcription of sv, probably indirectly through the action of a hypothetical repressor X. This inactivates the shaft differentiation program in the socket cell.