Abstract
An LCMSMS method for the simultaneous quantification of nicotine, cotinine, trans-3’-hydroxycotinine and norcotinine in human plasma was developed and fully validated. Potential endogenous and exogenous interferences were extensively evaluated and limits of quantification were determined by decreasing analyte concentration. Analytical ranges were 1–500 ng/mL for nicotine and cotinine, 5–500 ng/mL for trans-3’-hydroxycotinine and norcotinine. Mean intra- and inter-assay analytical recovery were between 101.9 and 116.8%, and intra- and inter-assay imprecision were less than 11% RSD for all analytes: parameters were evaluated at three different concentrations across the linear range of the assay. Extraction efficiency was ≥ 70% for all analytes. This validated method is useful for determination of nicotine and metabolites in human plasma to support research on the role of nicotine biomarkers on neuronal systems mediating cognitive and affective processes and to differentiate active, passive and environmental exposure.
Keywords: Nicotine, Cotinine, trans-3’-hydroxycotinine, Norcotinine, Plasma, LCMSMS
1. INTRODUCTION
Nicotine is the most abundant and potent pharmacological alkaloid in tobacco with behavioral effects of memory facilitation, locomotor activation, antinociception, mild calming, appetite suppression, and physiological effects of increased heart rate and blood pressure, changes in skin temperature, nausea and respiratory distress [1–5]. Nicotine is oxidized by hepatic cytochrome P450 2A6 to cotinine, that undergoes further oxidation to trans-3′-hydroxycotinine (3-OH-cotinine) and other metabolites [6, 7]. Previous investigators reported a relatively short plasma half-life (t1/2 = 1−2 h) for nicotine [8]; however, since cotinine and 3-OH-cotinine have longer plasma half-lives (18–20 h and 4– 8 h, respectively) than nicotine [8, 9], these are considered appropriate biomarkers for evaluating tobacco smoke exposure.
A number of methods have been developed to measure nicotine and metabolites in biological fluids including radioimmunoassay [10, 11], high performance liquid chromatography (HPLC) with ultraviolet detection [12–17] and gas chromatography [18, 19]. Most of these assays require a long chromatographic analysis time to achieve better separation of analytes of interest from matrix. In addition, GCMS assays required lengthy derivatization procedures. High performance liquid chromatography coupled with mass spectrometry (LCMS) is frequently the toxicologist’s method of choice for the quantification of drugs in biological matrices [20]. Gray et al. recently reported the quantification of nicotine and metabolites in meconium by LCMSMS [21, 22]. Kim and Huestis reported the quantification of nicotine, cotinine, 3-OH-cotinine and norcotinine in plasma by LCMS with limit of quantification (LOQ) of 2.5 ng/mL. [23]. Ghosheh et al. reported an LOQ of 10 ng/mL for nicotine and cotinine in plasma [24].
Our aim was to develop a method to simultaneously quantify nicotine and metabolites in human plasma to support our fMRI research on the role of nicotine in neuronal systems mediating cognitive and affective processes. This assay also will be useful to quantify nicotine biomarkers in smokers and non-smokers during active smoking, during nicotine withdrawal and during nicotine replacement therapy.
2. EXPERIMENTAL
2.1. Reagents
(R, S) 3-OH-cotinine (10 mg powder), 3-OH-cotinine -d3 (1 mg powder), (R,S)-norcotinine (10 mg powder) and (R,S)-norcotinine-d4 (5 mg powder) were purchased from Toronto Research Chemicals (North York, Ontario, Canada). (S)–Nicotine (1 mg powder) and formic acid were obtained from Sigma (St. Louis, MO, USA). (S)-Cotinine (1 mg/mL in methanol), (S)-cotinine-d3 (100 µg/mL in methanol) and (S)-nicotine-d4 (100 µg/mL in methanol) were acquired from Cerilliant (Austin, TX, USA). Water, acetonitrile, sodium phosphate dibasic, sodium phosphate monobasic, ammonium acetate, sodium acetate, hydrochloric acid, dichloromethane, 2-propanol, and ammonium hydroxide were from J.T. Baker (Philipsburg, NJ, USA) and methanol from Fisher Chemical (Pittsburgh, PA, USA). All solvents and reagents were HPLC or ACS grade. CleanScreen solid phase extraction (SPE) columns, part ZSDAU020, were purchased from United Chemical Technologies (Bristol, PA, USA). Different pools of blank human plasma were tested prior to use to ensure absence of analytes of interest or endogenous interferences.
Sodium phosphate buffer (pH 6.0 ± 0.05) was prepared with 0.1 M sodium monophosphate and 0.1 M sodium dibasic phosphate. Elution solvent (methylene chloride: isopropanol: concentrated ammonium hydroxide, 78:20:2 v/v/v) was prepared fresh daily.
2.2. Instrumentation
MS/MS analysis was performed using a MDS Sciex API 3200 QTrap® triple quadrupole/linear ion trap mass spectrometer with a TurboIonSpray source (Applied Biosystems, Foster City, CA, USA). The HPLC system consisted of Shimadzu LC-20AD pumps and SIL-20AC autosampler (Columbia, MD, USA). Analyst software version 1.4.1 was used for data analysis. Sonication was performed by a Branson 3510 Ultrasonicator (Danbury, CT, USA).
2.3. Preparation of calibrators, internal standards (IStd) and quality control (QC) samples
For calibrators, a stock solution of 100 µg/mL of each analyte was prepared in methanol and stored at −20°C until use. Working solutions, ranging from 10–5,000 ng/mL, were prepared by dilution with methanol. Blank human plasma specimens (200 µL) were fortified with 20 µL aliquots of working solutions to yield an eight-point calibration curve (1, 2.5, 5, 10, 50, 100, 250, and 500 ng/mL) for nicotine and cotinine, and six-point calibration curve (5, 10, 50, 100, 250, and 500 ng/mL) for 3-OH-cotinine and norcotinine. QC samples were prepared in a similar way from different stock solutions prepared from different vials. Blank human plasma specimens were fortified with 20 µL aliquots of QC working solutions to yield 4, 40, and 400 ng/mL for nicotine and cotinine, and 8, 40, and 400 for 3-OH-cotinine and norcotinine (low, medium, and high, respectively).
Individual deuterated IStd (nicotine-d4, cotinine-d3, 3-OH-cotinine -d3 and norcotinine-d4) stock 10,000 ng/mL solutions were prepared in methanol and stored at − 20°C until use. A working solution containing all IStd at a concentration of 1000 ng/mL was prepared in methanol. Ten µL of the IStd working solution was added to 200 µL of blank human plasma, yielding a final plasma concentration of 50 ng/mL. Quantification was accomplished by comparing peak area ratios of target analytes to IStd over all the concentrations across the linearity range. Data were fit to a linear least-squares regression curve with a weighting factor of 1/x.
2.4. Specimen preparation
Blank or authentic plasma (200 µL), as appropriate, standard or QC solution and 10 µL IStd solutions were pipetted into a 15 mL polypropylene tube. Two mL 2 M sodium acetate buffer, pH 6 was added and vortexed prior to SPE.
2.5. Solid phase extraction
CleanScreen DAU SPE columns were conditioned with 3 mL methanol, 3 mL water and 2 mL of 2 M sodium acetate buffer, pH 6. Samples were poured onto columns and allowed to flow by gravity alone. Columns were washed with 2 mL water, dried under vacuum for 1 min, followed by 1.5 mL 100 mM hydrochloric acid, drying for 5 min, and finally washed with 2 mL methanol followed by 5 min drying. Analytes were eluted with freshly prepared 5 mL dichloromethane:isopropanol:ammonium hydroxide (78:20:2, v/v/v). Eluates were dried under nitrogen at 40°C after addition of 100 µL 1% hydrochloric acid in methanol (v/v). Specimens were reconstituted in 200 µL water with 0.01% formic acid (v/v) and 30 µL was analyzed.
2.6. LCMSMS
Chromatographic separation was performed on a Synergi Hydro RP column (75 × 2.0 mm, 4 µm), protected by a guard column with identical packing material (4 × 2.0 mm; Phenomenex, Torrance, CA, USA). Isocratic elution occurred with (A) 1 mM ammonium acetate with 0.01% formic acid and (B) 0.01% formic acid in acetonitrile (35:65 v/v) at a flow rate of 400 µL/min. Mass spectrometric data were collected in positive ion mode, with optimized TurboIonSpray-MS parameter settings shown in Table 1. Source parameters were set to 206842.7 Pa curtain gas, 241316.5 Pa auxiliary gas, 275790.3 Pa nebulizer gas, medium collision gas, 3.0 µA nebulizer current, and 400°C source temperature after flow injection analysis source optimization with ultrapure nitrogen gas. Quadrupoles one and three were set to unit resolution for better sensitivity. The following transitions were monitored: m/z 163 to 132 and 84 for nicotine, m/z 177 to 80 and 98 for cotinine, m/z 193 to 80 and 134 for OH-cotinine, m/z 163 to 80 and 118 for norcotinine. The underlined transitions were the quantifier ions.
Table 1.
Liquid chromatography-tandem mass spectrometry parameters for nicotine and metabolites in plasma.
| Compounds | Q1 Mass (amu) |
Q3 Mass (amu) |
Dwell Time (msec) |
Declustering Potential (volts) |
Entrance Potential (volts) |
Collision Entrance Potential (volts) |
Collision Energy (volts) |
Cell Exit Potential (volts) |
Retention time (± SD) N= 30 |
|---|---|---|---|---|---|---|---|---|---|
| Nicotine | 163.2 | 132.2 | 150 | 35 | 5.5 | 12 | 21 | 4 | 0.950 (± 0.033) |
| 84.2 | 150 | 35 | 4.5 | 12 | 29 | 4 | |||
| Nicotine-d4 | 167.2 | 136.1 | 150 | 35 | 6 | 12 | 21 | 4 | 0.938 (± 0.027) |
| 121.0 | 150 | 35 | 6` | 12 | 29 | 4 | |||
| Cotinine | 177.2 | 98.1 | 150 | 41 | 3 | 12 | 29 | 4 | 0.860 (± 0.010) |
| 80.1 | 150 | 41 | 3 | 12 | 33 | 4 | |||
| Cotinine-d3 | 180.2 | 101.2 | 150 | 36 | 3 | 12 | 31 | 4 | 0.902 (± 0.008) |
| 80.2 | 150 | 36 | 3 | 12 | 33 | 4 | |||
| OH-Cotininea | 193.2 | 134.0 | 150 | 46 | 8 | 12 | 27 | 4 | 0.800 (± 0.025) |
| 80.2 | 150 | 46 | 8 | 12 | 35 | 4 | |||
| OH-Cotinine-d3 b | 196.2 | 134.1 | 150 | 46 | 10.5 | 14 | 27 | 4 | 0.796 (± 0.014) |
| 79.9 | 150 | 46 | 10.5 | 14 | 38 | 4 | |||
| Norcotinine | 163.2 | 118.2 | 150 | 46 | 10.5 | 12 | 29 | 4 | 0.830 (± 0.018) |
| 80.2 | 150 | 41 | 10 | 12 | 33 | 4 | |||
| Norcotinine-d4 | 167.2 | 139.2 | 150 | 46 | 10.5 | 12 | 29 | 4 | 0.840 (± 0.009) |
| 84.2 | 150 | 46 | 10.5 | 12 | 33 | 4 | |||
trans-3’-hydroxycotinine,
trans-3’-hydroxycotinine-d3
2.7. Data analysis
Peak area ratios of target analytes and respective IStd were calculated at each concentration. The most abundant transition for each analyte was used for quantification; the second transition served as a qualifier (Table 1).
2.8. Method validation
Selectivity, sensitivity, limits of detection (LOD) and limits of quantification (LOQ), linearity, imprecision, analytical recovery, extraction efficiency, matrix effect, carryover effect, dilution integrity, and stability were evaluated. Method validation was accomplished in four days with four unique assays.
Selectivity of the method was assessed by analyses of six plasma specimens from different pools. Each blank sample was extracted and analyzed for potential interferences from endogenous substances. In addition, potential interferences from commonly used drugs and minor tobacco alkaloids were evaluated by fortifying drugs into low-concentration QC samples. Final interferent concentrations were 1 µg/mL of cocaine, benzoylecgonine, norcocaine, norbenzoylecgonine, Δ9- tetrahydrocannabinol (THC), 11-hydroxy-THC, 11-nor-9-carboxy-THC, morphine, normorphine, morphine-3-beta-D-glucuronide, morphine-6-beta-D-glucuronide, codeine, norcodeine, 6-acetylmorphine, 6-acetylcodeine, hydrocodone, hydromorphone, oxycodone, noroxycodone, oxymorphone, noroxymorphone, diazepam, lorazepam, oxazepam, alprazolam, clonidine, ibuprofen, pentazocine, caffeine, diphenhydramine, chlorpheniramine, brompheniramine, aspirin, acetaminophen, phencyclidine, nitrazepam, flunitrazepam, temazepam, nordiazepam, amphetamine, methamphetamine, anabasine and anatabine. Each interference injection was followed by two blank samples injections. No interference was noted if all analytes quantified within ±20% of target concentrations. Specificity also was assessed by relative retention time and qualifier/quantifier transition peak area ratios. Transition peak area ratios for QC and authentic specimens were required to be within ± 20% of the mean calibrator transition peak area ratio.
Sensitivity of the method was evaluated by empirically determining the lowest concentration with a signal to noise ratio of at least 3:1 (LOD) and 10:1 (LOQ) for quantifier and qualifier transitions with acceptable chromatography and retention time. Linearity of the method was investigated by calculating the regression line by the method of least squares and expressed by the correlation coefficient (r2) and required to be ≥ 0.99. Method linearity was determined with at least 6 calibrators with a weighting factor of 1/x. Concentrations of each calibrator were required to be within ± 15% of target for calibrators when calculated against the full curve, except for the LOQ, for which ± 20% was acceptable.
Intra-day imprecision and analytical recovery were determined from five replicates at three different concentrations. Inter-day imprecision and analytical recovery were evaluated on five different days with four replicates on each day (n=20). Imprecision was expressed as % RSD of the calculated concentrations. The guidelines given by Krouwer and Rabinowitz [25] were followed for the calculation of imprecision. Analytical recovery was determined by comparing the mean result for all analyses to the nominal concentration value.
Extraction efficiency and matrix effect were evaluated on five different plasma lots with the three set system described by Matuszewski et al. [26]. In the first set, plasma samples were fortified with analytes and IStd prior to SPE. In set 2, plasma samples were fortified with analytes and IStd after SPE, and the third set contained “neat” analytes and IStd in mobile phase. There were 5 replicates in each set. Extraction efficiency, expressed as a percentage, was calculated by dividing mean peak areas of set 1 by set 2. Matrix effect was calculated by dividing the mean peak area of set 2 by set 3. The value was converted to a percentage and subtracted from 100 to represent the amount of signal suppressed by the presence of matrix. Acceptable carryover was defined as no quantifiable transition peaks in a blank plasma sample containing IStd immediately following a sample containing two times the upper LOQ. Dilution integrity was evaluated by diluting plasma samples (n=3) containing 1600 ng/mL of each analyte with blank plasma to achieve a 1:4 dilution. IStd was added and samples were extracted as described. Dilutional integrity was maintained if specimens quantified within ±20% of 400 ng/mL.
Stability was evaluated with human plasma fortified with analytes of interest at the three QC concentrations (n = 5). Short-term temperature stability was evaluated for human plasma stored for 24 h at room temperature, 72 h at 4°C, 72 h on the autosampler (15°C), and after three freeze-thaw cycles at −20°C. On the day of analysis, IStd was added to each specimen and analyzed as described. Calculated concentrations of stability specimens were compared to QC samples prepared on the day of analysis.
3. RESULTS AND DISCUSSION
Mass spectrometric (MS) optimization was performed by direct infusion of single analytes of interest. Optimized parameters and fragmentor voltages were chosen for each ion product to maximize sensitivity (Table 1). Quantification was based on the intensity of analyte molecular ions. Ions with the highest m/z value for each analyte and minimum background interferences were selected, providing better peak shapes for quantification. Linear calibration curves were obtained with a mean correlation coefficient (r2, weighting factor 1/x, n = 5) of > 0.99 for all analytes in human plasma (Table 2).
Table 2.
Nicotine and metabolites in plasma by liquid chromatography-tandem mass spectrometry: limits of detection (LOD), limits of quantification (LOQ), and calibration results (N=5).
| Analyte | LOD (ng/mL) |
LOQ (ng/mL) |
ULOQa (ng/mL) |
Y-intercept Mean ± SD |
Slope Mean ± SD |
R2 |
|---|---|---|---|---|---|---|
| Nicotine | 0.5 | 1 | 500 | −0.007098 ± 0.00925 |
0.9224 ± 0.07271 |
0.9991 |
| Cotinine | 0.5 | 1 | 500 | −0.007768 ± 0.17814 |
1.642 ± 0.15287 |
0.9988 |
| OH-Cotinineb | 2.5 | 5 | 500 | −0.04224 ± 0.01739 |
1.286 ± 0.10667 |
0.9978 |
| Norcotinine | 2.5 | 5 | 500 | −0.007712 ± 0.02125 |
0.7072 ± 0.09423 |
0.9983 |
Upper limit of quantification,
trans-3’-hydroxycotinine
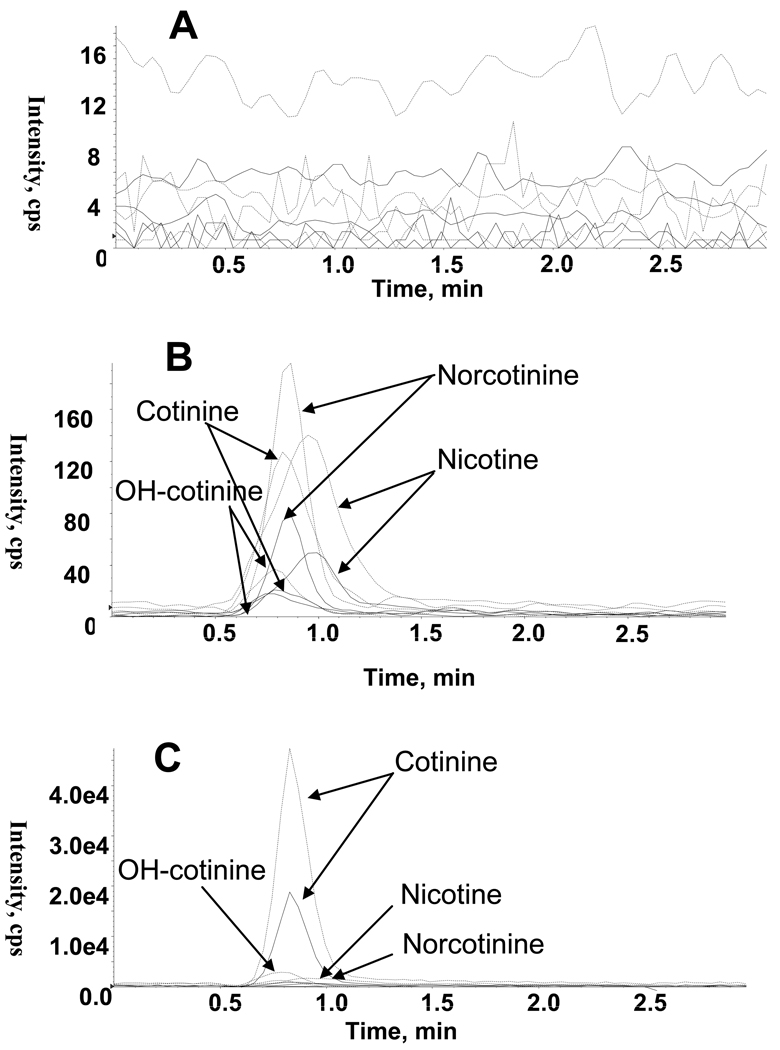
Total ion chromatograms for blank plasma, plasma fortified at the low QC concentration, and an authentic plasma specimen following controlled nicotine administration are shown in Figure 1. Analytes were eluted within 2 min (Figure 1), with a total chromatographic run time of 3 min. Empirical determination of LOQ with decreasing concentrations of analytes achieved LOQ of 1, 1, 5 and 5 ng/mL for nicotine, cotinine, norcotinine, and 3-OH-cotinine, respectively. Total ion chromatograms for analytes showed ≤75% interference from the plasma matrix. In addition, high recovery was achieved contributing to the low LOQ attained. Pretreatment of blank human plasma by SPE was sufficient to minimize matrix effect to less than 74.9% (<11% CV) for all analytes. Deuterated IStd, nicotine-d4, cotinine-d3, 3-OH-cotinine-d3 and norcotinine-d4, were employed to minimize loss during specimen preparation and to compensate for matrix ion suppression or enhancement.
Figure 1.
Extracted ion chromatograms for nicotine (m/z 132.2, 84.2), cotinine (m/z 80.1, 98.1), trans-3’-hydroxycotinine (m/z 80.2, 134), and norcotinine (m/z 80.2, 118.2). (A) Blank human plasma, (B) Blank human plasma fortified at the low quality control concentration for each analyte, and (C) Human plasma containing 44 ng/mL nicotine, 222 ng/mL cotinine, 112 ng/mL trans-3’-hydroxycotinine, and 20 ng/mL norcotinine. Each analyte represented by quantifier transition (broken line) and qualifier transition (solid line).
Stability of the chromatographic method was evaluated by calculating retention time variability. Percent relative variation for retention times was ≤ 0.15% for all analytes over 30 consecutive injections (Table 1).
No endogenous signal was observed in 10 blank human plasma specimens demonstrating selectivity of the method. Also, no interference was found for 43 commonly used over-the-counter and abused drugs added to low QC samples. These fortified low QC samples quantified within 20% of target with transition ratios within 20% of mean calibrator transition ratio. No drug interfered with quantification of any analyte of interest.
As shown in Table 3 intra-assay analytical recovery was determined by replicate analysis (n= 5) of the three QC samples. Intra-assay analytical recovery for all analytes was more than 109.2%. Inter-assay analytical recovery was assessed with 20 samples at each QC concentration on four separate runs and ranged from 102.6–115.1%. Intra-, inter-day and total assay imprecision at 3 QC concentrations determined from 20 different measurements with day as the grouping variable were <11% RSD. Extraction efficiencies for all analytes (n= 5) were estimated by comparing the LCMSMS peak area of unextracted and extracted samples. Mean extraction efficiencies for cotinine, 3-OH-cotinine, and norcotinine were 70.9–97.8%, and for nicotine 82.9–124.5% at three QC concentrations (Table 4). Blank plasma samples injected after the highest calibrator did not show carryover. The ability of the method to accurately quantify specimens containing high concentrations of analytes was evaluated by diluting 1600 ng/mL samples (n=3) with blank plasma to achieve a 1:4 dilution. All samples quantified within 16% of the target concentration of 400 ng/mL, confirming dilution integrity.
Table 3.
Mean ± SD intra- and inter-assay recovery and imprecision for nicotine and metabolites in plasma.
| Analyte | Expected conc. (pg/mg) |
Analytical recovery (% of target) | Imprecision (N=20, %RSD) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intra-assay N=5 | Inter-assay N=20 | Pooled Intra-day |
Inter-day | Total | |||||
| Mean | Range | Mean | |||||||
| 4 | 114.2 | (90.2–120) | 111.0 | 7.2 | 1.9 | 7.4 | |||
| Nicotine | 40 | 110.6 | (94.1–120) | 102.6 | 6.9 | 0.0 | 6.1 | ||
| 400 | 115.6 | (108–119) | 113.3 | 3.2 | 0.0 | 3.2 | |||
| 4 | 116.8 | (105–119) | 115.1 | 2.9 | 1.0 | 3.1 | |||
| Cotinine | 40 | 111.4 | (92.2–119) | 106.0 | 5.1 | 4.6 | 6.9 | ||
| 400 | 115.8 | (101–120) | 113.8 | 4.1 | 2.4 | 4.7 | |||
| 8 | 115.0 | (99.9–120) | 112.9 | 5.3 | 0.0 | 5.3 | |||
| OH-Cotininea | 40 | 112.0 | (86.2–120) | 101.9 | 7.8 | 7.3 | 10.7 | ||
| 400 | 116.4 | (95.2–119) | 110.4 | 5.3 | 5.0 | 7.3 | |||
| 8 | 109.2 | (89.1–117) | 104.6 | 8.5 | 3.2 | 9.1 | |||
| Norcotinine | 40 | 115.2 | (91.5–120) | 105.8 | 5.5 | 5.9 | 8.0 | ||
| 400 | 114.2 | (102–120) | 113.0 | 3.9 | 0.0 | 3.9 | |||
trans-3’-hydroxycotinine
Table 4.
Extraction efficiency and matrix effect for nicotine and metabolites extracted from plasma.
| Analyte | Extraction efficiency (%, N = 5) | Relative matrix effect (% suppressed, N = 5, %CV) | ||||
|---|---|---|---|---|---|---|
| Low | Medium | High | Low | Medium | High | |
| Nicotine Nicotine-d4 |
124.5 105.0 |
90.9 86.5 |
85.4 82.9 |
45.4 (7%) 74.9 (5%) |
45.3 (11%) 61.7 (5%) |
65.4 (10%) 62.7 (7%) |
| Cotinine Cotinine-d3 |
85.4 83.7 |
90.8 85.7 |
79.4 75.0 |
28.2 (8%) 37.6 (9%) |
27.9 (6%) 35.9 (6%) |
24.2 (6%) 31.5 (9%) |
|
trans-3’-Hydroxycotinine trans-3’-Hydroxycotinine-d3 |
86.8 77.8 |
84.4 81.5 |
73.5 70.9 |
48.8 (10%) 61.7 (8%) |
39.4 (7%) 57.6 (4%) |
59.9 (5%) 62.9 (6%) |
| Norcotinine Norcotinine-d4 |
97.8 94.0 |
93.7 92.2 |
81.8 79.3 |
35.0 (8%) 65.0 (5%) |
67.4 (3%) 56.9 (5%) |
57.1 (7%) 57.9 (3%) |
Analyte stability under four storage conditions was assessed. Losses of less than 17% were detected at room temperature for 24 h, 72 h on the autosampler, and after three freeze-thaw cycles. Refrigerated storage for 72 h should be avoided, as analyte losses were 6.5–32% of freshly extracted plasma specimens.
Extraction of nicotine and metabolites with liquid/liquid extraction (LLE) has been reported by several authors [27–32]. Solvent selection and evaporation are critical steps in nicotine-cotinine specimen preparation. LLE requires a large volume of organic solvent that must be evaporated with caution because of the high volatility of nicotine and metabolites. In order to reduce nicotine volatility, hydrochloric acid, acetic acid, phosphoric acid or sulphuric acid is added to form nicotine salts prior to evaporation [30, 33, 39–41]. SPE can simplify specimen preparation and reduce extraction time and solvent consumption as compared to LLE. SPE methods with Extrelut [33], Oasis HLB [34], Drug Test-1 [35], C18 Isolute [36], Bond Elut Certify [23], Clean Screen [37], and Amberlite XAD-2 [38] are available.
We previously reported simultaneous quantification of nicotine and metabolites from meconium with a LOQ of 5 ng/g for nicotine and nornicotine, and 1.25 ng/g for cotinine, OH-cotinine and norcotinine [21]. A Synergi Polar RP column with 150 mm × 4 µm was employed for the meconium analysis, while a Synergi Hydro RP column with 75 mm × 4 µm was utilized for the current plasma method. Analytes were required to be fully separated from meconium endogenous substances, necessitating a longer, more polar column. The total chromatographic run time for nicotine biomarkers in meconium was 12 min and in plasma 3 min. Furthermore, we achieved additional sensitivity for nicotine and cotinine of 1 ng/mL in the new plasma assay. In addition, the APCI source used in the analysis of nicotine in meconium could not achieve the sensitivity accomplished with the TurboIonSpray in plasma.
The current method improves on the previously reported plasma procedure by Kim and Huestis [23] in reducing specimen size from 1000 µL to 200 µL plasma. The above method was developed on a single quadruple mass spectrometer employing the precursor ion for quantification and selected ion monitoring (SIM) acquisition. No ion ratio was performed between the quantifier ion and the qualifier to confirm the presence of the analyte of interest. Also, the above method included only two deuterated internal standards (nicotine-d4 and norcotinine-d4) to quantify four analytes, nicotine, norcotinine, cotinine and 3-OH-cotinine. Furthermore, the chromatographic run time was 16 min. The present method was developed on a more sensitive triple quadruple MSMS instrumentation taking into consideration the international validation criteria. Two transition ions were selected as quantifier and qualifier, and the ion ratio between them was maintained below 20% across all method validation parameters. A more selective and sensitive acquisition method [Multiple reaction monitoring (MRM)] was chosen for quantification using deuterated compounds for each analyte of interest to minimize loss during specimen preparation and to compensate for matrix suppression or enhancement. The chromatographic run time was reduced to 3 min with method imprecision less than 11% RSD.
To demonstrate the applicability of the method, plasma concentrations of nicotine, cotinine, 3-OH-cotinine, and norcotinine in a tobacco smoker were determined 2–2.5 h after placement of a Nicoderm® nicotine patch (21 mg/24 h; GlaxoSmithKline, Moon Township, PA, USA) to the upper back [42]; concentrations were 44, 222, 112, and 20 ng/mL, respectively. Concentrations in the same subject 2–2.5 h after placement of a placebo patch were 4, 130, 62, and 7 ng/mL, respectively (Figure 1).
4. CONCLUSION
The current method improves on previously published LCMS methods for the simultaneous quantification of nicotine and metabolites in plasma by improving sensitivity, including a thorough method validation and clearly describing identification criteria and procedures to quantify analytes of interest.
ACKNOWLEDGMENT
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.U.S. Environmental Protection Agency, EPA/600/6-90/006F. 1992 [Google Scholar]
- 2.Aceto MD, Martin BM. Med. Res. Rev. 1982;2:43. doi: 10.1002/med.2610020104. [DOI] [PubMed] [Google Scholar]
- 3.Benowitz NL, Porchet H, Jacob P., III . Pharmacokinetics, metabolism and pharmacodynamics of nicotine. In: Wonnacott, et al., editors. Nicotine Psychopharmacology. Oxford: Oxford Univ.; 1990. [Google Scholar]
- 4.Imperato A, Mulus A, Di Chiara G. Eur. J. Pharmacol. 1986;132:337. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- 5.Henningfield JE, Goldberg SR. Pharmacol. Biochem. Behavior. 1983;19:989. doi: 10.1016/0091-3057(83)90405-7. [DOI] [PubMed] [Google Scholar]
- 6.Kyerematen GA, Vesell ES. Drug Metab. Rev. 1991;23:3. doi: 10.3109/03602539109029754. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima M, Yamamoto T, Nunoya K, Yokoi T, Nagashima K, Inoue K, Funae Y, Shimada N, Kamataki T, kuroiwa Y. Drug Metab. Dispos. 1996;24:1212. [PubMed] [Google Scholar]
- 8.Benowitz NL, Jacob P., III Clin. Pharmacol. Ther. 1994;56:483. doi: 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- 9.Benowitz NL, Jacob P., III Br. J. Clin. Pharmacol. 2001;51:53. doi: 10.1046/j.1365-2125.2001.01309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haines CF, Jr, Mahajan DK, Miljkove D. Clin. Pharmacol. Ther. 1974;16:1083. doi: 10.1002/cpt19741661083. [DOI] [PubMed] [Google Scholar]
- 11.Castro A, Monji N, Malkus H, Eisenhart W, Mckennis H, JR, Bowman ER. Clin. Chem. Acta. 95;1979:473. doi: 10.1016/0009-8981(79)90198-0. [DOI] [PubMed] [Google Scholar]
- 12.Parvianen MT, Barlow RD. J. Chromatogr. 1988;431:216. doi: 10.1016/s0378-4347(00)83088-1. [DOI] [PubMed] [Google Scholar]
- 13.Thuan NT, Migueres ML, Roche D. Clin. Chem. 1989;35:1456. [PubMed] [Google Scholar]
- 14.Rop PP, Grimaldi F, Oddoze C. J. Chromatogr. 1993;612:302. doi: 10.1016/0378-4347(93)80177-6. [DOI] [PubMed] [Google Scholar]
- 15.Zuccaro P, Altieri I, Rosa M. J. Chromatogr. B. 1995;668:187. doi: 10.1016/0378-4347(95)00066-r. [DOI] [PubMed] [Google Scholar]
- 16.Page-Sharp M, hale TW, Hackett LP. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003;796:173. doi: 10.1016/j.jchromb.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima M, Yamamoto T, Kuroiwa Y. J. Chromatogr. B Biomed. Appl. 2000;742:211. doi: 10.1016/s0378-4347(00)00149-3. [DOI] [PubMed] [Google Scholar]
- 18.James H, Tizabi Y, Tayler R. J. Chromatogr. B Biomed. Appl. 1998;708:87. doi: 10.1016/s0378-4347(97)00624-5. [DOI] [PubMed] [Google Scholar]
- 19.Jacob P, III, Wu S, Yu L. J. Pharm. Biomed. Anal. 2000;23:653. doi: 10.1016/s0731-7085(00)00343-5. [DOI] [PubMed] [Google Scholar]
- 20.Brewer E, Henion J. J. Pharm. Sci. 1998;87:395. doi: 10.1021/js9701059. [DOI] [PubMed] [Google Scholar]
- 21.Gray TR, Shakleya DM, Huestis MA. J. Chromatogr. B. 2008;863:107. doi: 10.1016/j.jchromb.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray TR, Magri R, Shakleya DM, Huestis MA. Clin. Chem. 2008;54:2018. doi: 10.1373/clinchem.2008.109173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim I, Huestis MA. J. Mass Spectom. 2006;41:815. doi: 10.1002/jms.1039. [DOI] [PubMed] [Google Scholar]
- 24.Ghosheh OA, Debra D, Rogers T, de Leon J, Dwoskin LP, Crooks PA. J. Pharma. Biomed. Anal. 2000;23:543. doi: 10.1016/s0731-7085(00)00339-3. [DOI] [PubMed] [Google Scholar]
- 25.Krouwer JS, Rabinowitz R. Clin. Chem. 1984;30:290. [PubMed] [Google Scholar]
- 26.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Anal. Chem. 2003;75:3019. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 27.Ceppa F, El Jahiri Y, Mayaudon H, Dupuy O, Burnat P. J. Chromatogr. B Biomed. Sci. Appl. 2000;746:15. doi: 10.1016/s0378-4347(00)00306-6. [DOI] [PubMed] [Google Scholar]
- 28.hariharan M, VanNoord T. Clin. Chem. 1991;37:1276. [PubMed] [Google Scholar]
- 29.Torano JS, Van Kan HJ. Analyst. 2003;128:838. doi: 10.1039/b304051h. [DOI] [PubMed] [Google Scholar]
- 30.Shin HS, Kim JG, Shin YJ, Jee SH. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002;769:177. doi: 10.1016/s1570-0232(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 31.Ji AJ, Lawson GM, Anderson R, dale LC, Croghan IT, Hurt RD. Clin. Chem. 1999;45:85. [PubMed] [Google Scholar]
- 32.James H, Tizabi Y, Taylor R. J. Chromatogr. B Biomed. Sci. Appl. 1998;708:87. doi: 10.1016/s0378-4347(97)00624-5. [DOI] [PubMed] [Google Scholar]
- 33.Tapani T, Tom J, Kari R. Clin. Chem. 1999;45:2164. [Google Scholar]
- 34.Xu X, Iba MM, Weisel CP. Clin. Chem. 2004;50:2323. doi: 10.1373/clinchem.2004.038489. [DOI] [PubMed] [Google Scholar]
- 35.Doctor PB, Gokani VN, Kulkarni PK, Parikh JR, Saiyed HN. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004;802:323. doi: 10.1016/j.jchromb.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Baidoo EE, Clench MR, Smith RF, Tetler LW. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003;796:303. doi: 10.1016/j.jchromb.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 37.kim I, William DD, Huestis MA. J. Chromatogr. B. 2005;814:233. doi: 10.1016/j.jchromb.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 38.Tyrpien K, Wielkoszynski T, Janoszka B, Dobosz C, Bodzek D, Steplewski Z. J. Chromatogr. A. 2000;870:29. doi: 10.1016/s0021-9673(99)01239-x. [DOI] [PubMed] [Google Scholar]
- 39.Van Vunakis H, Gjika HB, Langone JJ. IARC Sci. Publ. 1987;81:317. [PubMed] [Google Scholar]
- 40.Christiane O, Jean CD, Monique B. Clin. Chem. 1999;45:505. [Google Scholar]
- 41.Dietrich H, Ernst LW. Encyclopedia of Occupational Health and safety. (Fourth ed.) 2002 [Google Scholar]
- 42.Hahn B, Ross TJ, Wolkenberg FA, Shakleya DM, Huestis MA, Stein EA. Cereb. Cortex. 2008 Dec 10; doi: 10.1093/cercor/bhn226. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]