Abstract
Objective
The economic implications of sedative choice in the management of patients receiving mechanical ventilation are unclear because of differences in costs and clinical outcomes associated with specific sedatives. Therefore, we aimed to determine the cost-effectiveness of the most commonly used sedatives prescribed for mechanically ventilated critically ill patients.
Design, Setting, and Patients
Adopting the perspective of a hospital, we developed a probabilistic decision model to determine if continuous propofol or intermittent lorazepam was associated with greater value when combined with daily awakenings. We also evaluated the comparative value of continuous midazolam in secondary analyses. We assumed that patients were managed in a medical intensive care unit and expected to require ventilation for at least 48 hours. Model inputs were derived from primary analysis of randomized controlled trial data, medical literature, Medicare reimbursement rates, pharmacy databases, and institutional data.
Main Results
We measured cost-effectiveness as costs per mechanical ventilator-free day within the first 28 days after intubation. Our base-case probabilistic analysis demonstrated that propofol dominated lorazepam in 91% of simulations and, on average, was both $6,378 less costly per patient and associated with over three additional mechanical ventilator-free days. The model did not reveal clinically meaningful differences between propofol and midazolam on costs or measures of effectiveness.
Conclusion
Propofol has superior value compared to lorazepam when used for sedation among the critically ill who require mechanical ventilation when used in the setting of daily sedative interruption.
Keywords: cost-effectiveness, cost-benefit analysis (MeSH), critical illness (MeSH), respiration, artificial (MeSH)
INTRODUCTION
Mechanical ventilation is the most common intervention delivered daily to tens of thousands of patients in intensive care units (ICUs) worldwide. (1, 2) The provision of pharmacological sedation and analgesia to those receiving ventilation is an essential part of quality critical care because adequate sedation can reduce patient anxiety, improve patient-ventilator synchrony, and facilitate overall clinical care. (3, 4)
Current clinical practice guidelines recommend the provision of either intermittent or continuous lorazepam as the primary sedative for patients requiring prolonged ventilation and continuous propofol or midazolam for patients requiring short-term (48–72 hours) ventilation. (5) Although these and other sedatives have been compared in past studies, few have been assessed rigorously with high-quality, randomized clinical trials that included outcomes such as duration of ventilation and length of hospital stay. (6) To our knowledge, only two randomized clinical trials evaluating sedation regimens have been published that also incorporated daily trials of awakening from sedation, an intervention now widely utilized. (7, 8) Neither of these studies, one comparing midazolam to propofol and the other comparing propofol to intermittent lorazepam, reported a survival advantage to any specific sedative regimen. (7, 8)
Given the lack of mortality differences among these three sedatives, other factors such as costs and clinical outcomes like frequency of adverse events, duration of mechanical ventilation, and length of stay could be useful in differentiating the value of these products that are part of the $180 billion spent annually on critical care in the US. (7, 9–11) Although propofol may facilitate weaning when compared to lorazepam, it is significantly more costly than lorazepam and is associated with significant hemodynamic and metabolic side effects. (12, 13) Because of these concerns, many practitioners use midazolam because it is perceived to have a considerably shorter half-life than lorazepam and is less expensive than propofol. However, accumulation of midazolam and its active metabolites is likely to occur in critically ill patients potentially causing prolonged sedation. (14)
Because of the strong association between ICU length of stay and costs, identification of safe and effective sedative options that can also reduce resource utilization during a time of changing population demographics is important for policymakers and clinicians alike. (15, 16) However, we know of no formal economic analyses comparing sedatives in the setting of contemporary critical care practice. Therefore, we performed cost-effectiveness analysis comparing continuous propofol and intermittent lorazepam for the sedation of critically ill adults with secondary analyses including midazolam.
METHODS
Study design
We used a decision analysis model to compare the value of continuous propofol and intermittent lorazepam in our base-case analysis (Figure 1). In secondary analyses, we compared continuous midazolam to both propofol and lorazepam since they are the three most commonly used sedatives in an ICU setting. (17) We did not include midazolam as part of the primary analysis because of the lack of data on its direct comparison with lorazepam in the setting of daily awakening from sedation. We assumed that if patients tolerated the sedative, they then survived or died based on model inputs. Patients who experienced a sedative-associated adverse event crossed over to the comparator sedative, accruing costs and effects to the initial treatment while achieving the intended therapeutic effect with the second sedative.
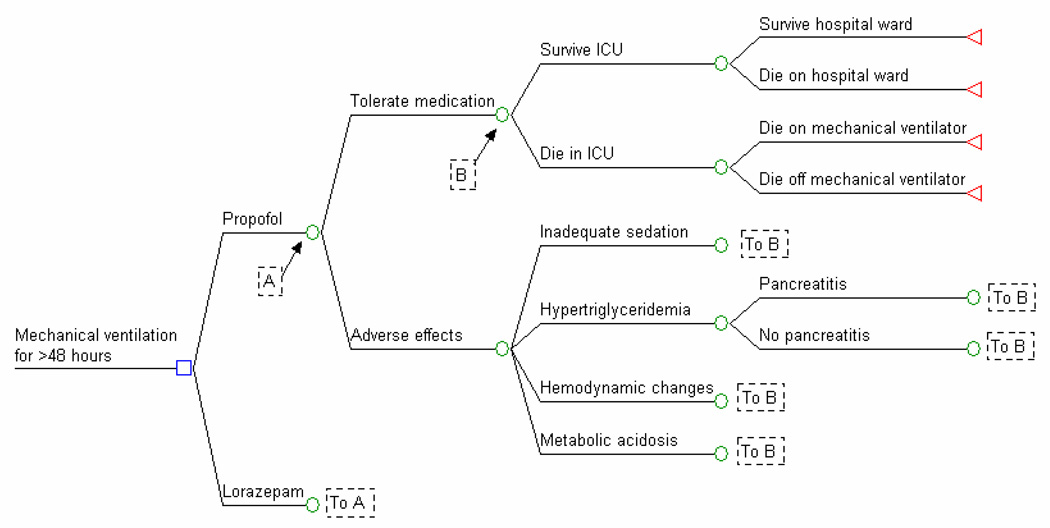
Figure 1. Decision Model.
This is a simplified version of the decision model used in analyses. In this model, patients ventilated for at least 48 hours could either receive propofol or lorazepam for sedation. Progression through the decision tree over the course of the succeeding 28 days examined was determined by probabilities defined in Table 1.
We followed recommendations for the conduct of cost-effectiveness analyses whenever possible, though with some notable departures due to the unique nature of the patient group and interventions compared. (18) First, instead of the recommended societal perspective, we conducted the analysis from the perspective of the hospital because prospective payment reimbursement structures in place between hospitals and payors create incentives for providers to minimize costs. Also, because there is no known long-term survival advantage to any particular sedation protocol, we limited the time horizon to 28 days from the initiation of mechanical ventilation. All costs were adjusted to 2007 US dollars using the Medical Care component of the US Consumer Price Index. (19) Because of the short time horizon, costs were not discounted.
Our primary data sources included original data from randomized controlled trials published by Carson and colleagues, comparing propofol to intermittent lorazepam, and Kress, et al, who randomized patients to propofol versus midazolam as well as to daily awakening trials versus no awakening. (7, 8) Patients were enrolled in these studies if they had an anticipated requirement for mechanical ventilation of >48 hours and required significant amounts of sedatives either in the form of continuous infusions or more than minimal doses of lorazepam. We also included data from what we felt were relevant, high quality studies (Table 1).
Table 1.
Input Variables and Sources for Base-Case Scenario and Ranges for Sensitivity Analyses
| Variable | Base-Case Estimate* |
Distribution | Range Used in Sensitivity Analyses |
Data Source |
|---|---|---|---|---|
| Clinical | ||||
| Medication utilization during mechanical ventilation | ||||
| Propofol, mg/day | 2,773 (290) | Gamma | 949–4,347 | (7) |
| Lorazepam, mg/day | 12 (2) | Gamma | 4–23 | (7) |
| Midazolam, mg/day | 54 (6) | Gamma | 34–84 | (8) |
| Morphine, mg/day | ||||
| Propofol base-case | 71 (14) | Gamma | 16–100 | (7) |
| Lorazepam recipient | 25 (5) | Gamma | (7) | |
| Midazolam recipient | 42 (5) | Gamma | 14–80 | (8) |
| Medication failure leading to crossover† | ||||
| Propofol | ||||
| Inadequate sedation | 4% (3%) | Beta | 0–25% | (7) |
| Hypertriglyceridemia‡ | 1% (1%) | Beta | 0–15% | (12) |
| Pancreatitis‡ | 0% (1%) | Beta | 0–10% | (12) |
| Hemodynamic instability | 1% (1%) | Beta | 0–10% | (12) |
| Lorazepam | (7) | |||
| Inadequate sedation | 8% (3%) | Beta | 0–25% | |
| Metabolic acidosis | 0% (1%) | Beta | 0–5% | |
| Hospital mortality | ||||
| Propofol | 37% (6%) | Beta | 5–75% | (7, 8) |
| ICU during mechanical ventilation | 64% | |||
| ICU after extubation | 12% | |||
| Hospital ward after ICU discharge | 24% | |||
| Lorazepam | 37% (6%) | Beta | 5–75% | (7) |
| ICU during mechanical ventilation | 75% | |||
| ICU after extubation | 4% | |||
| Hospital ward after ICU discharge | 21% | |||
| Length of care | ||||
| Ventilator days | Gamma | (7, 8) | ||
| Propofol, all patients | 6 (2) | |||
| Propofol survivor | 4 (2) | 2–21 | ||
| Propofol decedent | 7 (2) | 2–21 | ||
| Lorazepam, all patients | 8 (2) | |||
| Lorazepam survivor | 9 (2) | 2–21 | ||
| Lorazepam decedent | 7 (2) | 2–21 | ||
| ICU length of stay | Gamma | (7, 8) | ||
| Propofol survivor | 9 (2) | 5–28 | ||
| Propofol decedent | 9 (2) | 5–28 | ||
| Lorazepam survivor | 12 (2) | 7–28 | ||
| Lorazepam decedent | 9 (2) | 5–28 | ||
| Hospital length of stay | 18 (3) | Gamma | 10–30 | (7, 8) |
| Additional ICU days with mechanical ventilation for complications | ||||
| Pancreatitis | 2 | Fixed | 0–5 | Assumption |
| Metabolic acidosis | 1 | Fixed | 0–2 | Assumption |
| Costs | ||||
| Medications | ||||
| Propofol (10mg/mL; 100mL) | $22.77 | Fixed | $11.37–$60.77 | (24) |
| Lorazepam (2mg single injection) | $1.82 | Fixed | $0.91–$7.28 | (24) |
| Midazolam (5mg/mL) | $1.21 | Fixed | $0.60–$7.37 | (24) |
| Morphine (2mg single injection) | $1.03 | Fixed | $0.80–$3.70 | (39) |
| Other | ||||
| Serum triglyceride level | $7.90 | Fixed | $4–$12 | Institutional data¶ |
| Serum lipase level | $7.24 | Fixed | $4–$18 | Institutional data¶ |
| Plastic tubing for infusion | $5.50 | Fixed | $2–$8 | Institutional data¶ |
| CT of abdomen & pelvis with contrast | $312 | Fixed | $156–$468 | (27) |
| Intensive care unit | (25, 26, 40) | |||
| Mechanical ventilation, day 1 | $6,325 | Fixed | $1,825–$9,488 | |
| Mechanical ventilation, day 2 | $5,076 | Fixed | $1,825–$7,614 | |
| Mechanical ventilation, ≥ day 3 | $4,431 | Fixed | $1,825–$6,647 | |
| No mechanical ventilation, day | $3,311 | Fixed | $1,825–$4,967 | |
| Hospital | ||||
| Hospital ward, day | $1,261 | Fixed | $631–$1,892 | (26, 40) |
| Physician** | (27) | |||
| ICU care/day, intubated | $246.26 | Fixed | $123.13–$369.39 | |
| ICU care/day, extubated | $76.81 | Fixed | $38.31–$115.22 | |
| Hospital ward care/day | $54.04 | Fixed | $27.02–$81.06 | |
CT=computerized tomography, hr=hour, ICU=intensive care unit, kg=kilogram, mcg=microgram, mg=milligram, mL=milliliter Propofol and midazolam assumed to have clinical equivalence with the exceptions of side effect profile (assumed to mirror lorazepam) and costs.
Number is parentheses represents standard error. For point estimates presented as percentages, the sample size was 132 (64 in lorazepam group and 68 in propofol group).
Costs were added for propofol recipients who developed hypertriglyceridemia (two extra serum triglyceride levels) and propofol-associated pancreatitis (daily lipase level while in the ICU, one abdomen and pelvis computerized tomography with and without contrast, and two extra ICU days with ventilation). Lorazepam recipients who developed an associated metabolic acidosis were assumed to require one extra ventilator day.
Hypertriglyceridemia was defined as serum triglyceride level >500mg/dL while pancreatitis was defined as either serum amylase ≥125 IU/L or lipase ≥60 IU/L.
Based on costs from Duke University Medical Center.
We assumed ICU day one required >104 minutes of critical care time (CPT 99291 + 99292), other ICU days with intubation required 30-74 minutes of critical care time (CPT 99291), and both post-extubation ICU care (CPT 99233) and hospital ward care (CPT 99232). (27)
Patient population and process of care characteristics
Base-case patient
We modeled the base-case patient on the cohort composition reported in the Carson, et al study in which patients were 55 years old and managed in a medical intensive care unit. We also assumed patients would receive screening daily for the appropriateness of spontaneous breathing trials in anticipation of ventilator liberation and that daily awakening trials were performed in which sedatives were stopped each morning until patients could follow simple commands.
Sedative and analgesic dosing
Estimates of sedative and analgesic dosing were based on original data from the Carson and Kress studies (Table 1). Sedative doses were targeted to achieve a Ramsay sedation score of 3 (responds to command only) or 4 (asleep but with a brisk response to a light glabellar tap or loud sound) in the Kress study and 2 (cooperative, oriented, and tranquil) or 3 (responds to command only) in the Carson study. (20) The protocols for dosing continuous propofol, continuous midazolam, intermittent lorazepam, and morphine have been described elsewhere. (7, 8)
Adverse events
We recognized that the sedative initially prescribed might either provide inadequate sedation or lead to untoward adverse effects, necessitating crossover to the comparator drug. For the base-case analysis, we assumed that patients who did not tolerate propofol crossed over to lorazepam and vice versa. In secondary analyses in which midazolam was included, we assumed that propofol-intolerant patients received midazolam and vice versa. Expenses related to the adverse effects of sedatives are shown in Table 1.
Overall base-case crossover rates (6% for propofol and 8% for lorazepam) were based on Carson, et al. We assumed that the failure rate for midazolam would approximate that of lorazepam (8%). Adverse effects of propofol (inadequate sedation, hypertriglyceridemia, pancreatitis, and hemodynamic instability) as well as lorazepam (inadequate sedation and metabolic acidosis related to the drug’s propylene glycol carrier) and midazolam (inadequate sedation) were included in the model and their input values in sensitivity analyses defined by ranges reported in the relevant literature. (12, 13, 21, 22)
Equivalence of comparisons between midazolam and propofol recipients
Our model assumed that clinical outcomes (though not drug costs) of those receiving midazolam were equivalent to propofol recipients based on the study by Kress, et al. (8) This study reported no significant differences in clinical characteristics or outcomes including length of stay and mortality between propofol and midazolam recipients whom received daily awakenings from sedation. We independently verified this equivalency through our own statistical analyses of the original Kress, et al dataset (not shown).
Clinical effects
We expressed our results in terms of costs and both mechanical ventilator-free days and mechanical ventilator-free survival. Mechanical ventilator-free days were defined as the total number of days free from mechanical ventilation within the first 28 days from the time of intubation. Mechanical ventilator-free survival was defined as the total number of days free from mechanical ventilation within the first 28 days from the time of intubation for hospital survivors. The validity and utility of using ventilator-free days as an outcome in critical care research has been described elsewhere. (23)
Hospital Outcomes
Because there is no proven survival difference between those receiving propofol, lorazepam, or midazolam, we assumed that ICU and hospital mortality were equivalent in all groups. Further, we assumed that the average duration of hospitalization was equal for all groups based on the work of Carson, et al. The primary differences among groups were related to length of ICU stay and duration of mechanical ventilation, outcomes that differed based on ICU survivorship status. (7, 8)
Costs
Only direct costs were incorporated in the model (Table 1). Although costs were estimated using a variety of sources relevant to different stakeholders in this issue, we incorporated published costs from national database sources when possible to maximize the ability to generalize our results. We held unit costs static in the base-case probabilistic model, though varied important cost variables in one-way sensitivity analyses.
Pharmacy
Drug prices were representative of 2007 national wholesale acquisition costs (WACs) to provide better estimates of the actual cost borne by a hospital system compared to the use of average wholesale prices. (24) We have included in our one-way sensitivity analyses wide variance in drug costs to account for the potential diversity in actual costs encountered by different providers. In general, the cost range examined in sensitivity analyses represents +/−50% of the WAC with the exception that propofol’s upper bound corresponded to the WAC for the branded product Diprivan™.
Hospitalization
Daily ICU costs were based on the medical subset of patients drawn from a larger national sample of 51,000 critically ill ventilated patients. (25) A lower range for ICU cost in sensitivity analyses was the daily Medicare reimbursement rate for DRG 565 (mechanical ventilation for ≥96 hours). This was calculated by dividing the average DRG payment ($28,837) by its corresponding mean length of stay (15.8 days). The upper range was established by inflating the base-case daily ICU cost by 50%. Daily hospital ward costs were based on MedPAR analyses of general ICU patients utilizing the Russell equation and cost ranges used in sensitivity analyses were calculated by adding and subtracting 50% of the base-case cost. (26) We included professional costs for the primary treating physician based on current procedural terminology (CPT) codes and Medicare fee schedules, varying these costs +/− 50% in sensitivity analyses. (27)
Probabilistic and Sensitivity Analyses
Because of the uncertainty in some of our base-case estimates, we elected to present our main results based on probabilistic analyses generated using Monte Carlo simulation rather than deterministic analyses that would have generated only point estimates. Specifically, for probabilistic analyses, we first specified distributions for all input variables. We used gamma distributions to model continuous variables and beta distributions to model dichotomous variables (Table 2). The sample size (n= 132) used to calculate standard errors for probability distributions was based on number of patients enrolled in the Carson, et al study. (7) Next, average values of 1,000 separate simulations were reported along with cost-effectiveness scatterplots. We also performed one-way and two-way sensitivity analyses to understand how varying data inputs across pre-specified ranges (not distributions) could affect point estimates of costs and effectiveness (Table 2). We used Excel (Microsoft) to develop the cost-effectiveness analysis and Stata 9 (Stata; College Station, TX) for statistical analyses. These analyses were judged exempt from formal IRB review by 45 CFR 46.101(b) because they utilized previously collected, completely de-identified data.
Table 2.
Incremental Cost-effectiveness Ratios
| Scenario | Costs | Increase in Cost |
MVFD | Gain in MVFD |
MV-Free Survival |
Gain in MV-Free Survival |
Incremental Cost-Effectiveness of Regimen |
|
|---|---|---|---|---|---|---|---|---|
| $ | $ | $/MVFD Gained | $/MVFD Alive Gained |
|||||
| Base-case analysis | ||||||||
| Probabilistic analysis (crossover to lorazepam)* | ||||||||
| Lorazepam | 52,009 | 12.183 | 11.563 | Propofol dominates lorazepam in 91% of simulations |
||||
| Propofol | 45,631 | (6,378) | 15.917 | (3.734) | 14.956 | (3.393) | ||
| Secondary analyses | ||||||||
| Probabilistic analysis (crossover to midazolam)† | ||||||||
| Lorazepam | 52,954 | 12.803 | 12.297 | Propofol dominates lorazepam in 92% of simulations |
||||
| Propofol | 44,740 | (8,214) | 16.172 | (3.369) | 15.338 | (3.041) | ||
| Probabilistic analysis including midazolam | ||||||||
| Lorazepam | 55,038 | 12.126 | 11.46 | |||||
| Propofol | 44,969 | (10,069) | 15.156 | (3.03) | 14.324 | (2.864) | Propofol dominates lorazepam in 92% of simulations |
|
| Midazolam | 43,908 | (1,061) | 15.402 | 0.246 | 14.428 | 0.104 | Midazolam dominates lorazepam in 94% of simulations |
|
MVFD = mechanical ventilator-free days (days without mechanical ventilation within the 28 days following intubation), MV = mechanical ventilation. Parentheses denote negative value.
Assumes crossover to lorazepam for propofol intolerance and vice versa.
Assumes crossover to midazolam for propofol intolerance and to propofol for lorazepam intolerance.
RESULTS
In our base-case scenario, we found that propofol clearly dominated lorazepam based on its comparatively lower overall costs ($45,631 vs $52,009) and greater effects, as quantified by a gain of over three mechanical ventilator-free days (Table 2). In fact, propofol was less costly or more efficacious in 94% and 90% of 1,000 simulations performed, respectively. In 91% of simulations, propofol was both less costly and more efficacious.
Our secondary probabilistic analyses demonstrated that allowing propofol crossover to midazolam instead of lorazepam in the setting of associated adverse events produced results similar to the base-case (Table 2). Additionally, midazolam’s dominance of intermittent lorazepam in direct comparison was consistent with the base-case analysis comparing propofol to lorazepam. We found no meaningful differences between propofol and midazolam on costs or effectiveness, however. The superimposed clustering of 1,000 comparative simulations shown in Figure 2 and Figure 3 demonstrates that the true difference in value between the two is unlikely to be clinically important.
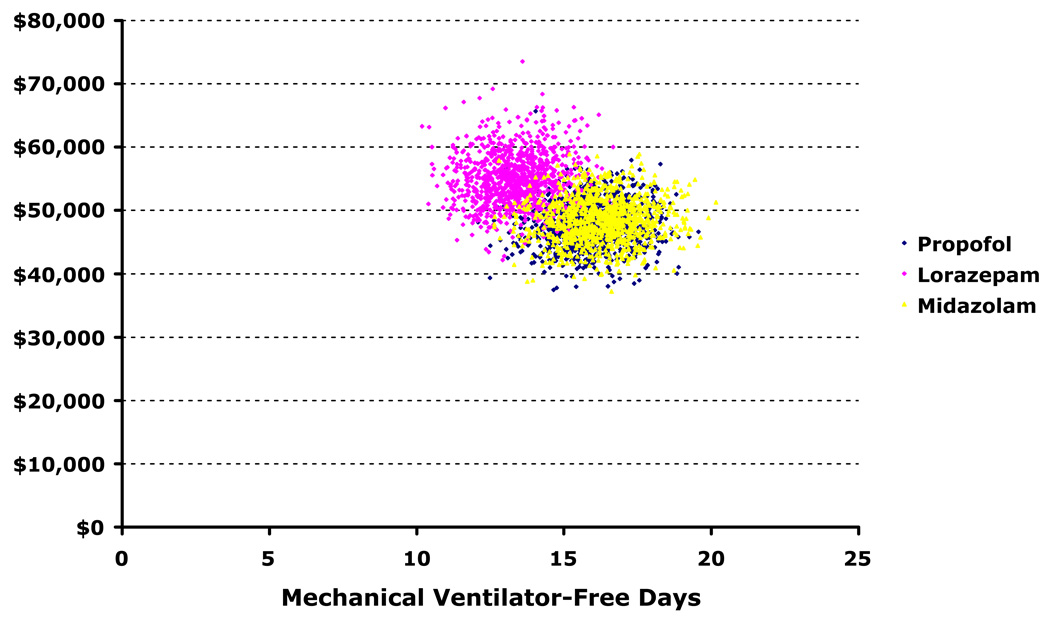
Figure 2. Scatterplot of probabilistic analyses comparing costs and effects.
These scatterplots depict the results of 1,000 simulations during which important clinical variables were allowed to vary simultaneously within predefined distributions (see Methods). In contrast to Figure 4, the axes represent the actual (not incremental) costs and effects of each separate trial. This figure demonstrates near duplication of propofol (blue circles) costs and effects for midazolam (yellow circles), though little overlap of lorazepam with either of these groups—emphasizing the dominance of propofol and midazolam over lorazepam (pink circles).
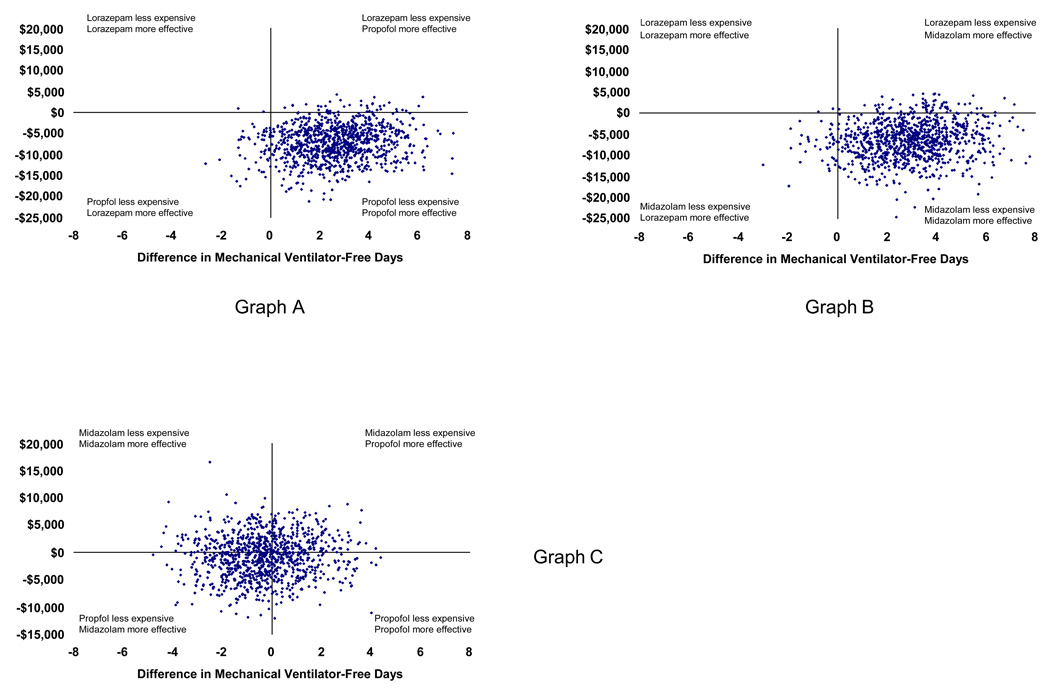
Figure 3. Scatterplot of probabilistic analyses comparing incremental costs and effects.
These scatterplots graph the results of 1,000 simulations during which important clinical variables were allowed to vary simultaneously within predefined distributions (see Methods). The x axis represents the incremental difference in mechanical ventilator-free days by group (propofol – lorazepam in Graph A, midazolam – lorazepam in Graph B, and propofol – midazolam in Graph C). The y axis represents incremental costs calculated in a similar fashion. The clustering of simulation results in the lower right quadrant in Graphs A and B demonstrates the comparative dominance of propofol and midazolam over lorazepam. The clustering of analyses at the intersection of the axes in Graph C suggests that there is little evidence that clinically or economically important differences exist between propofol and midazolam.
Although we found little evidence to favor propofol over midazolam, we were interested in exploring how robust our analyses were when comparing the two sedatives. Table 3 shows the results of varying average duration of ventilation by group since this variable appeared to have the greatest impact on sedative value in our model. Assuming clinical, not cost, equivalence in these two sedatives there is a minor cost advantage per case seen with midazolam ($319) relative to propofol. However, varying the difference in average ventilation duration by as little as one day had important effects on costs and ventilator-free days. For example, if propofol use is actually associated with one fewer day of ventilation relative to midazolam, overall comparative costs are $6,286 less with an average gain of 0.354 ventilator-free days. The opposite effect occurred when we assumed that midazolam was associated with a one day reduction in ventilator days compared to propofol.
Table 3.
One-way Sensitivity Analyses Comparing Propofol and Midazolam
| Average Difference in Ventilation Duration, Days | ||||||
|---|---|---|---|---|---|---|
| Propofol | 4 | 5 | 6 | 6 | 6 | |
| Midazolam | 6 | 6 | 6 | 5 | 4 | |
| ICU Costs | ||||||
| Propofol | 27,044 | 32,595 | 38,147 | 37,792 | 37,437 | |
| Midazolam | 37,437 | 37,729 | 38,147 | 32,595 | 27,044 | |
| Hospital Ward Costs | ||||||
| Propofol | 6,209 | 7,626 | 9,042 | 8,952 | 8,861 | |
| Midazolam | 8,861 | 8,952 | 9,042 | 7,626 | 6,209 | |
| Sedative Costs | ||||||
| Propofol | 383 | 470 | 557 | 555 | 553 | |
| Midazolam | 227 | 232 | 238 | 204 | 169 | |
| Total Costs | ||||||
| Propofol | 33,636 | 40,690 | 47,745 | 47,299 | 46,851 | |
| Midazolam | 46,525 | 46,976 | 47,426 | 40,423 | 33,421 | |
| Incremental costs | −12,889 | −6,286 | 319 | 6,876 | 13,430 | |
| MVFDs | ||||||
| Propofol | 16.684 | 16.305 | 15.926 | 15.951 | 15.975 | |
| Midazolam | 15.975 | 15.951 | 15.926 | 16.305 | 16.684 | |
| Incremental effects | 0.709 | 0.354 | 0 | −0.354 | −0.709 | |
| ICERs | propofol | propofol | midazolam | midazolam | Midazolam | |
| dominates | dominates | less costly | dominates | dominates | ||
ICU = intensive care unit, MVFD = mechanical ventilator-free days, ICER = incremental cost-effectiveness ratio.
Assumes baseline average of 6 days of mechanical ventilation.
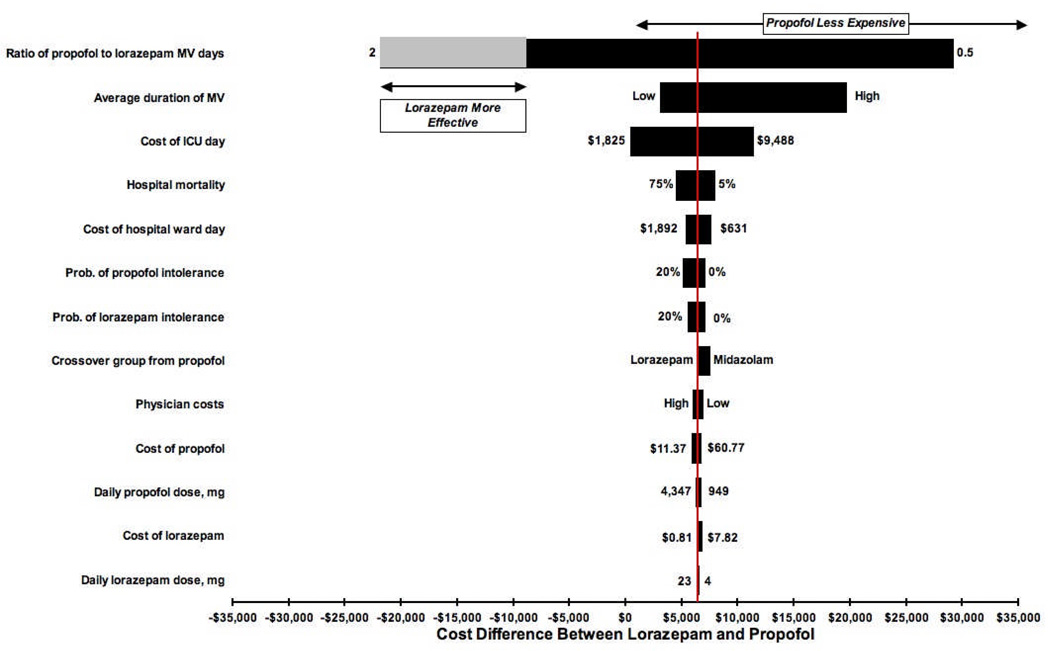
Across the range of one-way sensitivity analyses, propofol use was associated not only with gains in the number of ventilator-free days but also with lower costs in comparison with lorazepam as shown in the tornado diagram (Figure 4). The only realistic scenario in which lorazepam was more effective than propofol was when the propofol to lorazepam ratio of ventilator days exceeded 1.5. For example, assuming propofol use resulted in an average ventilation duration of 6 days, lorazepam use would theoretically result in greater numbers of ventilator-free days only when the average duration of ventilation for this group was less than 4 days (that is, 6 days/1.5) owing to the comparatively longer duration of ventilation and ICU care among lorazepam survivors of ventilation. Drug and physician costs had a minor impact on overall costs and effects in these sensitivity analyses.
Figure 4. Tornado diagram comparing cost differences between lorazepam and propofol.
The length of the horizontal bars corresponds to the difference in average costs between lorazepam and propofol groups over the range specified for variables of interest depicted on the y axis. The vertical line transecting the bars represents the cost difference between lorazepam and propofol in the point-estimate (non-probabilistic) base-case analysis. The horizontal line at the top of the figure represents the range of values over which propofol’s cost is less than that of lorazepam. There were fewer mechanical ventilator-free days in all ranges and scenarios with the exception of a propofol to lorazepam ratio of mechanical ventilation duration >1.5 (for example, 6 propofol ventilator days to 4 lorazepam ventilator days). The grey bar and horizontal arrow depict this gain in lorazepam-associated mechanical ventilator-free days.
DISCUSSION
Clinicians’ practice of prescribing and monitoring the use of sedatives among the hundreds of thousands of patients receiving mechanical ventilation in ICUs annually has profound implications. Sedation use is intimately associated not only with patient comfort but the duration of mechanical ventilation, incidence of delirium, and overall resource utilization. (28, 29) Because of the comparatively high cost of each ventilator day and the observation that many ICU patients may generate a net financial loss primarily related to ICU length of stay, healthcare providers should search preferentially for interventions and therapies designed to decrease days of ventilation. (26)
Given the advancing age of the US population and expected increase in critical care utilization in the coming decades, planning for future needs in a climate of potential economic restraint and resource limitation is crucial in the resource-intensive ICU setting. (30) Therefore, the results of our economic analysis have contemporary importance for physicians, pharmacists, administrators, and policymakers and add to the relatively small body of critical care cost-effectiveness research. (15, 31)
We believe that there is a common perception that propofol use is inherently expensive in comparison to lorazepam and that favoring the latter in routine clinical practice may conserve resources. (32) However, our analyses show that propofol has superior value when compared to lorazepam and may have equivalent value to midazolam when daily interruption of sedation occurs. We found that in nearly all scenarios and simulations, the use of propofol for sedation is associated with lower overall costs and greater ventilator-free days when compared to lorazepam. In fact, despite lorazepam’s considerably lower pharmacy unit cost, its use results in additional days of mechanical ventilation. This in turn, increases the risk of ventilator-associated pneumonia and other costly and morbid time-dependent hazards of critical care. (33) We feel that this and other studies suggest that using lorazepam for the sedation of mechanically ventilated patients who require more than minimal amounts of sedation conveys few advantages. (29)
It is difficult to compare our work to the existing medical literature examining sedatives from an economic perspective because these past studies were undertaken before clinical practice advanced to include daily awakenings from sedation and spontaneous breathing trials. It is likely that without daily interruption of continuous infusions, the difference in effectiveness between propofol or midazolam and lorazepam would be less. Additional limitations of past work include incorporation of medication costs instead of overall hospitalization costs, data collection within different countries during times when comparative exchange rates are difficult to now appreciate, consideration of only short-term sedation, lack of sensitivity analysis use to examine the impact of data input uncertainty, and inclusion of propofol costs based on its more expensive branded formulation. (32, 34–37)
Because of the ubiquity of sedative use in the ICU setting, we believe that our results underscore the importance of determining whether clinically important differences exist among commonly used sedatives with respect to mechanical ventilation-free days and length of stay. For example, assuming equivalence in ventilator-free days and length of stay, a hospital treating 1,000 ventilated patients annually could theoretically save over $300,000 by using midazolam instead of propofol. However, if midazolam use was actually associated with even one extra ICU day, the balance would swing in the opposite direction, with a comparative annual opportunity loss of over $2.5 million. Even an extra hospital ward day would represent over $385,000 in additional costs. The tens of thousands of ventilated patients managed daily magnify the potential implications of these unclear outcomes differences. Interestingly, our analyses suggest that even newer, more expensive sedatives such as dexmedetomidine (average cost $300–500 per day) are likely to have favorable value in comparison to other sedatives if their primary or adjunctive use is associated with a comparative reduction in either ICU or hospital length of stay by as little as one day. (38)
Our study has limitations that are important to highlight. First, we have utilized data from different sources. Although we gathered these data from what we felt were the highest quality studies, there may be bias inherent in this practice. Another factor worth considering is our use of data from two different trials to estimate values for key variables in the model for propofol and midazolam. Even though we performed our own analyses of these studies’ primary data to establish that no significant differences existed between these groups’ clinical outcomes (not shown), there could nonetheless be an underappreciated difference. Still, when we applied various ranges of input variables and outlined assumptions that seemed reasonable given the absence of a larger comparative randomized trial, our results were upheld. Third, our metric of cost per mechanical ventilator-free day is relatively novel in the cost-effectiveness literature. However, its use as an outcome measure in critical care is increasingly accepted and practical considering its salutary effect on sample sizes in clinical trials. (23) Also, because the impact of transitioning from one sedative to a less expensive alternative as the summative days of ventilation accrue is unknown, we were unable to include this strategy in our model. Also, we were unable to incorporate the independent effect of sedative-associated delirium because of source data limitations. (29) Finally, because the effect of different sedative regimens on long-term outcomes and costs is unknown, our model was focused on acute care outcomes. Although we believe our model is helpful for clinicians caring for typical critically ill patients, it is intended to augment and not replace clinical judgment in decision-making.
Conclusion
In conclusion, continuous propofol used for sedation among those receiving mechanical ventilation have significantly lower overall costs and a greater number of ventilator-free days in comparison to intermittent lorazepam. The routine use of lorazepam as the primary sedative choice should be discouraged based on its comparatively poor value in this particular critical care setting. Contemporary, adequately powered comparative studies of common sedatives including propofol and midazolam are also needed to determine if either has a comparative economic advantage.
ACKNOWLEDGMENTS
Christopher Cox had full access to all of the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis. No authors have conflicts of interest associated with the subject of this manuscript.
Financial support:
This research was supported by National Institutes of Health grants K23 HL081048 (CEC), K23 GM63906 K23 (JPK), and HL067068 (SSC). This includes the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript. The funding source had no role in the approval of the manuscript.
REFERENCES
- 1.Angus DC, Shorr AF, White A, et al. Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations. Crit Care Med. 2006;34(4):1016–1024. doi: 10.1097/01.CCM.0000206105.05626.15. [DOI] [PubMed] [Google Scholar]
- 2.Behrendt CE. Acute respiratory failure in the United States: incidence and 31-day survival. Chest. 2000;118(4):1100–1105. doi: 10.1378/chest.118.4.1100. [DOI] [PubMed] [Google Scholar]
- 3.Rotondi AJ, Chelluri L, Sirio C, et al. Patients' recollections of stressful experiences while receiving prolonged mechanical ventilation in an intensive care unit. Crit Care Med. 2002;30(4):746–752. doi: 10.1097/00003246-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 4.van de Leur JP, van der Schans CP, Loef BG, et al. Discomfort and factual recollection in intensive care unit patients. Crit Care. 2004;8(6):R467–R473. doi: 10.1186/cc2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30(1):119–141. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Ostermann ME, Keenan SP, Seiferling RA, Sibbald WJ. Sedation in the intensive care unit: a systematic review. JAMA. 2000;283(11):1451–1459. doi: 10.1001/jama.283.11.1451. [DOI] [PubMed] [Google Scholar]
- 7.Carson SS, Kress JP, Rodgers JE, et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit Care Med. 2006;34(5):1326–1332. doi: 10.1097/01.CCM.0000215513.63207.7F. [DOI] [PubMed] [Google Scholar]
- 8.Kress JP, Pohlman AS, O'Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342(20):1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 9.Pandharipande P, Pun B, Herr D, et al. American Thoracic Society International Conference. San Francisco, CA: 2007. May 21, Double blind randomized controlled trial comparing dexmedetomidine vs. lorazepam to reduce duration of delirium and coma in mechanically ventilated patients. [abstract] [Google Scholar]
- 10.Wittbrodt ET. Analysis of pharmacoeconomics of sedation and analgesia. Crit Care Clin. 2001;17(4):1003–1013. doi: 10.1016/s0749-0704(05)70191-3. [DOI] [PubMed] [Google Scholar]
- 11.Pronovost PJ. Care in the ICU: teaming up to improve quality. Presented at: Improving Quality of Care in Intensive Care Units Through Best Practices; September 4, 2002; Washington, D.C.: 2002. Available online at: http://www.kaisernetwork.org. Accessed July 28, 2007. [Google Scholar]
- 12.Devlin JW, Lau AK, Tanios MA. Propofol-associated hypertriglyceridemia and pancreatitis in the intensive care unit: an analysis of frequency and risk factors. Pharmacotherapy. 2005;25(10):1348–1352. doi: 10.1592/phco.2005.25.10.1348. [DOI] [PubMed] [Google Scholar]
- 13.Vasile B, Rasulo F, Candiani A, Latronico N. The pathophysiology of propofol infusion syndrome: a simple name for a complex syndrome. Intensive Care Med. 2003;29(9):1417–1425. doi: 10.1007/s00134-003-1905-x. [DOI] [PubMed] [Google Scholar]
- 14.Malacrida R, Fritz ME, Suter PM, Crevoisier C. Pharmacokinetics of midazolam administered by continuous intravenous infusion to intensive care patients. Crit Care Med. 1992;20(8):1123–1126. doi: 10.1097/00003246-199208000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Dorman T, Pauldine R. Economic stress and misaligned incentives in critical care medicine in the United States. Crit Care Med. 2007;35:S36–S43. doi: 10.1097/01.CCM.0000252911.62777.1E. [DOI] [PubMed] [Google Scholar]
- 16.Angus DC, Kelley MA, Schmitz RJ, White A, Popovich J., Jr Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population? JAMA. 2000;284(21):2762–2770. doi: 10.1001/jama.284.21.2762. [DOI] [PubMed] [Google Scholar]
- 17.Mehta S, Burry L, Fischer S, et al. Canadian survey of the use of sedatives, analgesics, and neuromuscular blocking agents in critically ill patients. Crit Care Med. 2006;34(2):374–380. doi: 10.1097/01.ccm.0000196830.61965.f1. [DOI] [PubMed] [Google Scholar]
- 18.Torrance GW, Siegel JE, Luce BR. Framing and designing the cost-effectiveness analysis. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. pp. 54–81. [Google Scholar]
- 19.Consumer price indexes. Available online at: http://www.bls.gov/cpi/home.htm. Accessed March 30, 2007.
- 20.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2(5920):656–659. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrientos-Vega R, Sanchez-Soria MM, Morales-Garcia C, Cuena-Boy R, Castellano-Hernandez M. Pharmacoeconomic assessment of propofol 2% used for prolonged sedation. Crit Care Med. 2001;29(2):317–322. doi: 10.1097/00003246-200102000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Wilson KC, Reardon C, Theodore AC, Farber HW. Propylene glycol toxicity: a severe iatrogenic illness in ICU patients receiving IV benzodiazepines: a case series and prospective, observational pilot study. Chest. 2005;128(3):1674–1681. doi: 10.1378/chest.128.3.1674. [DOI] [PubMed] [Google Scholar]
- 23.Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30(8):1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Average wholesale costs. Available online at: http://www.analysource.com. Accessed January 10, 2007.
- 25.Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266–1271. doi: 10.1097/01.ccm.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- 26.Cooper LM, Linde-Zwirble WT. Medicare intensive care unit use: analysis of incidence, cost, and payment. Crit Care Med. 2004;32(11):2247–2253. doi: 10.1097/01.ccm.0000146301.47334.bd. [DOI] [PubMed] [Google Scholar]
- 27.Current procedural terminology online. Available online at: https://catalog.ama-assn.org/Catalog/cpt/cpt_search.jsp. Accessed March 25, 2007.
- 28.Al-Haddad M, Hayward I, Walsh TS. A prospective audit of cost of sedation, analgesia and neuromuscular blockade in a large British ICU. Anaesthesia. 2004;59(11):1121–1125. doi: 10.1111/j.1365-2044.2004.03961.x. [DOI] [PubMed] [Google Scholar]
- 29.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Ward NS, Levy MM. Rationing and critical care medicine. Crit Care Med. 2007;35(2 Suppl):S102–S105. doi: 10.1097/01.CCM.0000252922.55244.FB. [DOI] [PubMed] [Google Scholar]
- 31.Talmor D, Shapiro N, Greenberg D, Stone PW, Neumann PJ. When is critical care medicine cost-effective? A systematic review of the cost-effectiveness literature. Crit Care Med. 2006;34(11):2738–2747. doi: 10.1097/01.CCM.0000241159.18620.AB. [DOI] [PubMed] [Google Scholar]
- 32.McCollam JS, O'Neil MG, Norcross ED, Byrne TK, Reeves ST. Continuous infusions of lorazepam, midazolam, and propofol for sedation of the critically ill surgery trauma patient: a prospective, randomized comparison. Crit Care Med. 1999;27(11):2454–2458. doi: 10.1097/00003246-199911000-00022. [DOI] [PubMed] [Google Scholar]
- 33.Cook DJ, Walter SD, Cook RJ, et al. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med. 1998;129(6):433–440. doi: 10.7326/0003-4819-129-6-199809150-00002. [DOI] [PubMed] [Google Scholar]
- 34.Swart EL, van Schijndel RJ, van Loenen AC, Thijs LG. Continuous infusion of lorazepam versus midazolam in patients in the intensive care unit: sedation with lorazepam is easier to manage and is more cost-effective. Crit Care Med. 1999;27(8):1461–1465. doi: 10.1097/00003246-199908000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Barrientos-Vega R, Mar Sanchez-Soria M, Morales-Garcia C, et al. Prolonged sedation of critically ill patients with midazolam or propofol: impact on weaning and costs. Crit Care Med. 1997;25(1):33–40. doi: 10.1097/00003246-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 36.MacLaren R, Sullivan PW. Pharmacoeconomic modeling of lorazepam, midazolam, and propofol for continuous sedation in critically ill patients. Pharmacotherapy. 2005;25(10):1319–1328. doi: 10.1592/phco.2005.25.10.1319. [DOI] [PubMed] [Google Scholar]
- 37.Anis AH, Wang XH, Leon H, Hall R. Economic evaluation of propofol for sedation of patients admitted to intensive care units. Anesthesiology. 2002;96(1):196–201. doi: 10.1097/00000542-200201000-00034. [DOI] [PubMed] [Google Scholar]
- 38.Siobal MS, Kallet RH, Kivett VA, Tang JF. Use of dexmedetomidine to facilitate extubation in surgical intensive-care-unit patients who failed previous weaning attempts following prolonged mechanical ventilation: a pilot study. Respir Care. 2006;51(5):492–496. [PubMed] [Google Scholar]
- 39.Drug Topics Redbook. Oradell, NJ: Medical Economics Co.; 2006. [Google Scholar]
- 40.Halpern NA, Pastores SM, Greenstein RJ. Critical care medicine in the United States 1985-2000: An analysis of bed numbers, use, and costs. Crit Care Med. 2004;32(6):1254–1259. doi: 10.1097/01.ccm.0000128577.31689.4c. [DOI] [PubMed] [Google Scholar]