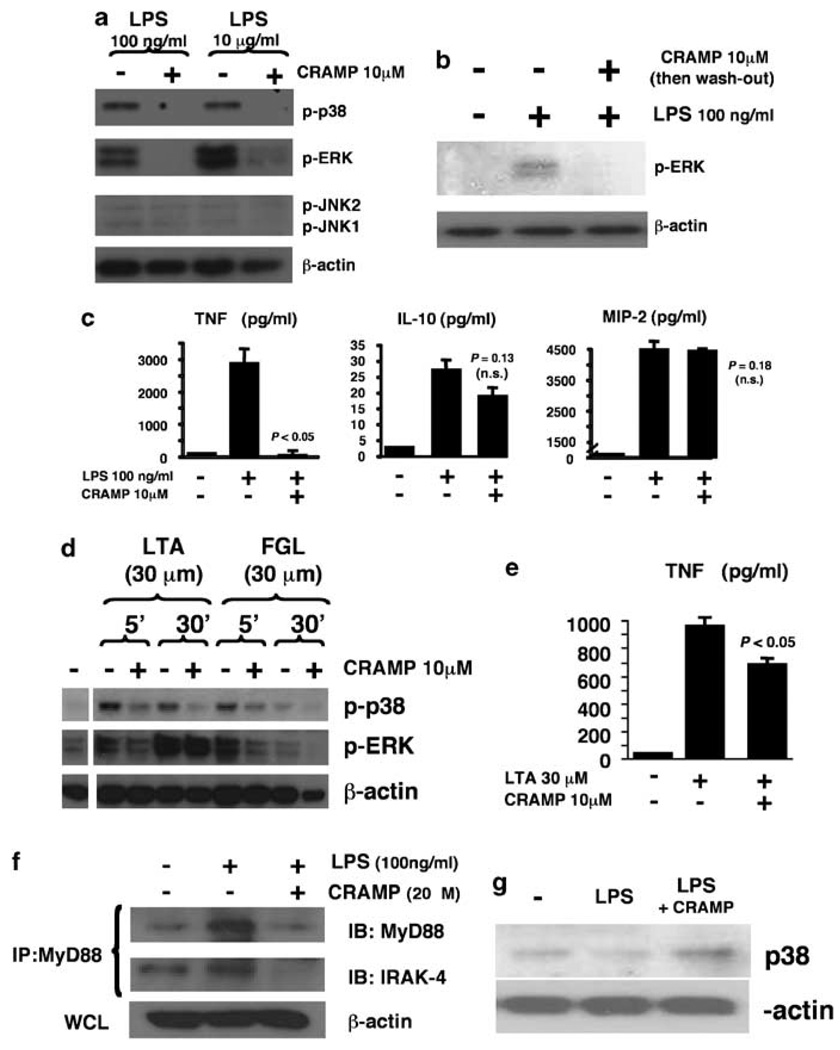
Figure 1.
Exogenous cathelicidin inhibits macrophage mitogen-activated protein kinase (MAPK) activation in response to lipopolysaccharide (LPS) and other toll-like receptor ligands. (a) Wild-type (WT) mouse bone marrow-derived macrophages (BMDM) were stimulated with LPS in the presence or absence of murine cathelicidin CRAMP; phosphorylation of MAPKs was assessed by western immunoblot. (b) WT BMDM stimulation experiment as mentioned above, but CRAMP removed by extensive washing before LPS stimulation and assessment of pERK stimulation. (c) WT mouse BMDM was stimulated with LPS for 3h in the presence or absence of CRAMP; tumor necrosis factor-α (TNFα), interleukin (IL)-12 and macrophage inflammatory protein-2 (MIP-2) release in supernatant determined by enzyme-einked emmunosorbent assay (ELISA), triplicate wells ± s.d. (d) WT mouse BMDM stimulated with lipoteichoic acid or flagellin in the presence or absence of CRAMP; phosphorylation of MAPK assessed by western immunoblot. (e) WT mouse BMDM stimulated with lipoteichoic acid in the presence or absence of CRAMP; TNFα levels measured by ELISA, mean of triplicate wells+s.d. All experiments were carried out three times with similar results; representative experiment are shown. (f) WT mouse BMDM were stimulated with LPS in the presence or absence of CRAMP. Cell lysates were immunoprecipitated with anti-MyD88 antibody and MyD88 and IL-1 receptor-associated kinase (IRAK)-4 expression were detected by western immunoblot. (g) WT mouse BMDM were primed overnight with LPS (100ng ml−1). On the second day, cells were restimulated with LPS for 3 h in the presence or absence of 10 µM CRAMP; p38 phosphorylation was detected by western immunoblot.