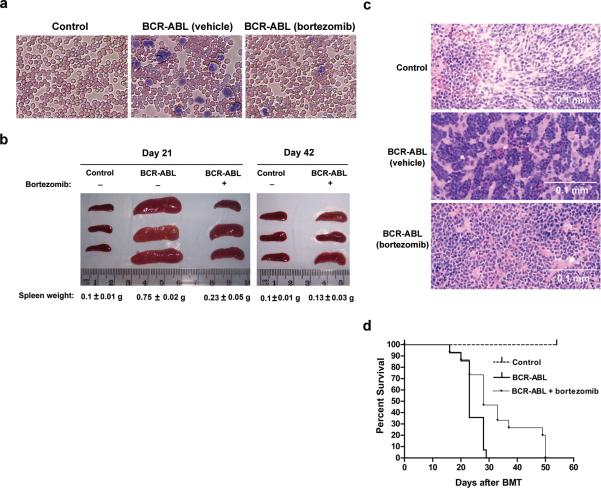
Figure 4. Treatment of BCR-ABL-transduced mice with bortezomib decreases signs of CML-like illness and prolongs survival.
a) Wright-Giemsa staining of blood smears from vehicle-treated vector control, vehicle-treated BCR-ABL and bortezomib-treated BCR-ABL-transduced mice (on day 21 after BMT, and after 4 treatments of 0.8mg/kg bortezomib) show an excess of granulocytes (dark purple) in BCR-ABL mice but decreased staining of granulocytes in bortezomib-treated BCR-ABL-transduced mice, comparable to vector control mice. Original magnification, 200X. b) Bortezomib treatment reduces splenomegaly in BCR-ABL-transduced mice. Spleens were isolated on day 21 after BMT and 4 bortezomib treatments (left panel) and on day 42 after BMT and 11 treatments (right panel). Groups treated with vehicle only are marked as (-), and groups treated with bortezomib are marked as (+). Spleen weight was indicated at the bottom of each group. The statistical significance of differences among various groups of spleen weight was determined by analysis of variance (ANOVA). N = 3 for all groups. Significant values are as follows: p < 0.001 for Day 21 BCR-ABL group treated with vehicle only versus all other groups (including Day 42 groups); p < 0.05 for Day 21 BCR-ABL group treated with bortezomib versus control groups; p < 0.05 for Day 42 BCR-ABL group treated with bortezomib versus Day 21 BCR-ABL group treated with bortezomib. c) H & E staining of spleen samples obtained from the same groups of mice described in (b) shows reduced myeloid infiltration in the spleens of BCR-ABL-transduced mice treated with bortezomib. d) Bortezomib treatment prolongs survival of BCR-ABL-transduced mice. BCR-ABL-transduced mice were treated with either vehicle (0.9% saline) or bortezomib (0.8mg/kg). Survival of mice was plotted using the Kaplan-Meier method (nonparametric cumulated survival fraction plot), and statistical comparison between the curves obtained using the log rank test. p < 0.01 for BCR-ABL group treated with bortezomib (n = 15) versus BCR-ABL group treated with vehicle only (n = 14).