Abstract
To quantify the reactions of nitric oxide (NO) with hemoglobin under physiological conditions and to test models of NO transport on hemoglobin, we have developed an assay to measure NO–hemoglobin reaction products in normal volunteers, under basal conditions and during NO inhalation. NO inhalation markedly raised total nitrosylated hemoglobin levels, with a significant arterial–venous gradient, supporting a role for hemoglobin in the transport and delivery of NO. The predominant species accounting for this arterial–venous gradient is nitrosyl(heme)hemoglobin. NO breathing increases S-nitrosation of hemoglobin β-chain cysteine 93, however only to a fraction of the level of nitrosyl(heme)hemoglobin and without a detectable arterial–venous gradient. A strong correlation between methemoglobin and plasma nitrate formation was observed, suggesting that NO metabolism is a primary physiological cause of hemoglobin oxidation. Our results demonstrate that NO–heme reaction pathways predominate in vivo, NO binding to heme groups is a rapidly reversible process, and S-nitrosohemoglobin formation is probably not a primary transport mechanism for NO but may facilitate NO release from heme.
In the last several years, the interactions of nitric oxide (NO) and hemoglobin have become the subject of much interest. A model has been recently proposed by Stamler and colleagues suggesting that NO is transported on hemoglobin by binding to the highly conserved β-chain cysteine 93 residue, forming S-nitrosohemoglobin (SNO-Hb) (1–4). According to this theory, NO carried on hemoglobin β-cysteine 93 is delivered from the lungs to the microvasculature where, on deoxygenation in the tissues, the S-nitroso linkage is weakened. This allows the NO molecules, free or complexed to small thiols, to diffuse through the erythrocytes to the vascular walls, causing vasodilation of the microvasculature and subsequent recruitment of more erythrocytes. The model also posits that in the venous circulation, deoxygenated hemoglobin preferentially binds NO at the heme group to form nitrosyl(heme)hemoglobin [Hb(FeII)NO], as total NO-modified hemoglobin levels were found to be equal in the arterial and venous blood of rats (2). However, recent studies argue that NO either is not released from SNO-Hb during deoxygenation (5) or that any NO released from SNO-Hb is actively scavenged by oxyhemoglobin and fails to affect vascular tone (6). Furthermore, to date in vivo SNO-Hb measurements have been performed only in the Sprague–Dawley rat, thus the physiological significance in humans has been questioned.
The reaction of NO with hemoglobin to form SNO-Hb is in addition to two other well-characterized NO reactions: with oxyhemoglobin to form methemoglobin (FeIII) and nitrate ion and with deoxyhemoglobin to form Hb(FeII)NO. This second reaction is also the subject of much current research. Yonetani and his research group and Kosaka and colleagues have shown that Hb(FeII)NO is a six-coordinate species (iron binds four nitrogens, a proximal histidine, and NO) and that on deoxygenation of the other heme groups, the proximal histidine bond stretches or breaks, forming a five-coordinate species with a lowered oxygen affinity (7–11). Hb(FeII)NO also demonstrates an enhanced Bohr effect, promoting oxygen release and increasing sensitivity to tissue acidosis (7). Despite the loss of an oxygen-binding site (replaced by NO), overall Hb(FeII)NO may therefore more effectively deliver oxygen than pure oxyhemoglobin to regions with very low oxygen tensions (10).
Although of potential biological importance, the amount of Hb(FeII)NO formation in the body has been uncertain because of limitations in the sensitivity of electron paramagnetic resonance, the primary method for measuring this hemoglobin species. A recent reanalysis of in vitro NO–oxyhemoglobin reactions, under relatively physiologic conditions of oxygen saturation, ionic strength, and molar ratios, has suggested that this pathway may be equal in magnitude to the long-studied reaction of NO oxidation of ferrous to ferric heme (12). Indeed, in vitro-measured levels of Hb(FeII)NO, SNO-Hb, and FeIII appeared comparable. These results are surprising for two reasons: first, the very high association rates and low dissociation rates of NO for both oxy- and deoxyheme groups suggest that heme nitrosylation and FeIII formation are expected to compete strongly with S-nitrosation of cysteine 93, and second, the relative abundance of oxyhemoglobin (99%) in these experiments would be expected to favor the reaction with oxyhemoglobin to form FeIII and nitrate rather than Hb(FeII)NO.
To clarify the role of these three mechanisms, by which NO can react with hemoglobin under physiological conditions, and to test these competing models of NO transport on hemoglobin in humans, we have developed a highly specific and sensitive assay for measuring total nitrosylated hemoglobin as well as individual levels of Hb(FeII)NO and SNO-Hb. Because the basal levels of nitrosylated hemoglobin in the human circulation are very low, we attempted to augment NO binding to hemoglobin by delivering NO by inhalation. It has been argued that the observed physiological effects of inhaled NO support models of NO transport and delivery on hemoglobin (12, 13). NO breathing has been demonstrated to reduce systemic vascular resistance (14, 15), increase kidney glomerular filtration (16), increase intestinal blood flow after ischemia reperfusion injury or NO synthase inhibition (17, 18), and increase aortic cGMP levels (19), indicating systemic effects. Thus, measurements of NO–hemoglobin reactions during NO inhalation in human volunteers represent a rigorous test of these competing models, uniting NO and physiological oxyhemoglobin/deoxyghemoglobin mixtures in the pulmonary circulation.
Materials and Methods
Protocol.
The study protocol was approved by the National Heart Lung and Blood Institute's Institutional Review Board, and all subjects signed an informed consent. All volunteers had a normal hemoglobin concentration, and their hemoglobin A was documented by electrophoresis. Volunteers fasted after midnight and during the study day. Peripheral i.v. and radial artery catheters were placed, and blood samples were collected hourly. After an hour of baseline measurements, subjects breathed NO gas at a concentration of 80 ppm (INOvent Delivery System, INO Therapeutics, Clinton, NJ). A room air gas condenser with an oxygen blender was used to deliver an FIO2 of 0.21 at 40 liters/min with an in-line reservoir bag. NO gas was delivered in line, and concentrations of NO, oxygen, and nitrogen dioxide (NO2) were sampled at the mask to ensure a delivery of 80 ppm NO and an FIO2 of 0.21 and to maintain the NO2 level < 1.0 ppm. The NO was discontinued after 2 h, and room NO levels dropped to <20 parts per billion within 10 min.
Ozone-Based Chemiluminescent Detection of Hb(FeII)NO and SNO-Hb.
The method for the measurement of nitrite and S-nitrosothiols by reaction with I3− to release NO gas (20, 21) was applied to hemoglobin that had been pretreated with and without KCN and K3Fe(CN)6. Seven milliliters of glacial acetic acid and 2 ml of distilled water were mixed with 50 mg of KI (or NaI). A crystal of I2 was added to yield a concentration of 6–20 mM (20). Helium was bubbled through the reaction mixture, through 1 N NaOH, and then into the Sievers Model 280 NO analyzer (Boulder, CO). Injection of samples into this I3− reactant stoichiometrically releases NO from nitrite, nitrosothiols, Hb(FeII)NO, and SNO-Hb with a sensitivity down to 1 pmol (see Results).
Blood samples were drawn and centrifuged at 750 × g for 5 min. Plasma was removed and stored at −70°C until assayed for nitrate/nitrite/S-nitrosothiols. The red blood cell pellet was removed and washed two times in five volumes of PBS. Red cell samples in duplicate were then lysed in both 1:4 dilution of 0.5 mM EDTA in nitrite-free molecular biology grade water (Biofluids, Bethesda, MD) or 0.2 M KCN and 0.2 M K3Fe(CN)6 in 0.5 mM EDTA distilled water. After 30-min incubation, 500 μl was passed through a 9.5-ml bed volume Sephadex G25 column to remove nitrite, small thiols, and KCN/K3Fe(CN)6. The hemoglobin concentration of the Sephadex G25 effluent was measured by conversion to cyanomethemoglobin (ɛ540 = 11 for heme). Hemoglobin samples (200 μl) were immediately drawn into 250-μl Hamilton syringes and reacted with I3− to release NO for chemiluminescent detection. The value for total nitrosylated hemoglobin was obtained from the reaction of hemoglobin without KCN/K3Fe(CN)6 in I3−, Hb(FeII)NO was obtained from the difference of the reaction of hemoglobin without KCN/K3Fe(CN)6 in I3− from the reaction with KCN/K3Fe(CN)6, and the value of SNO-Hb was obtained from the value of the reaction of hemoglobin with KCN/K3Fe(CN)6 pretreatment. The concentration (in nanomolar) of NO released was calculated by subtracting the NO concentration generated by 200-μl injection of the water from the Sephadex G25 column (background control). This control sample was obtained immediately before running the hemoglobin sample on the column. This value was divided by the concentration of the hemoglobin, and the total multiplied by 100 (expressed as percent mol nitrosylated hemoglobin per mol heme subunit).
Ozone-Based Chemiluminescent Determination of Serum Nitrate, Nitrite, and Low Molecular-Weight S-nitrosothiols.
Plasma samples were thawed and filtered through a prewashed 10,000 molecular weight cutoff filtration unit. Samples were then injected in different reductants in line with 1 N NaOH and the Sievers Model 280 NO analyzer. Nitrite was measured by reduction in acidified KI (7 ml of glacial acetic acid/2 ml of distilled water/50 mg of KI). Nitrite and S-nitrosothiols were measured in I3− (20). S-nitrosothiols were also measured in Cu+/l-cysteine (22). Nitrate was measured by reduction in vanadium(III) at 90°C (23). All of these assays demonstrated linear sensitivity above 1.0 pmol (r2 > 0.99, P < 0.001, for nitrite, nitrate, and SNO–glutathione).
FeIII Concentration.
FeIII concentrations were measured by using Co-Oximetry (CIBA–Corning 270 Co-Oximeter, Medfield, MA) immediately (less than 5-min delay) after obtaining venous and arterial samples from the volunteers. Measurements were validated by comparison with measurements by absorption spectroscopy at 700, 630, 576, and 560 nm by using the Winterbourn equation (24).
Statistical Analysis.
Two-tailed paired t tests were used to analyze increases in the mean values of paired samples before, during, and after NO inhalation, as well as to analyze differences in nitrosylated hemoglobin levels between arterial and venous blood at baseline and during NO breathing. Data are reported as the mean ± SEM.
Results
Arterial and Venous Total Nitrosylated Hemoglobin Before and During NO Breathing.
Because of the very low levels of nitrosylated hemoglobin in the human circulation, a technique was needed that is sensitive to levels of 1 × 10−5 (0.001%) mol of NO bound to hemoglobin per mol of heme subunit (0.004% with respect to hemoglobin tetramer). These levels are more than an order of magnitude lower than can be detected by electron paramagnetic resonance, a technique sensitive only for Hb(FeII)NO. We previously used a reductive assay by using I3− to release NO gas from freshly purified hemoglobin for detection in a chemiluminescent NO analyzer in three patients with sickle cell anemia and found arterial–venous gradients in total NO bound to hemoglobin during NO breathing (21).
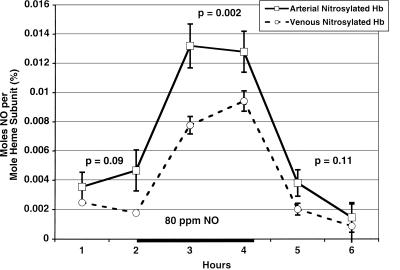
This methodology was used to measure the total NO bound to hemoglobin from red cells in the arterial and venous circulation of five normal volunteers (n = five normal volunteers) before and during NO breathing. Before NO inhalation, we found a trend toward an arterial–venous difference in total NO bound to hemoglobin with mean basal arterial levels of 0.004 ± 0.0008% and venous levels of 0.002 ± 0.0005% (P = 0.091). During NO breathing, there was a significant increase, compared with baseline, in mean arterial levels to 0.013 ± 0.001% (P = 0.001) and venous levels to 0.009 ± 0.0005% (P < 0.001), and a significant arterial–venous gradient was evident (P = 0.002). These values returned to baseline within 1 h after stopping NO inhalation (Fig. 1).
Figure 1.
Baseline and augmented levels of total nitrosylated hemoglobin (NO on either the heme or cysteine 93) in the arterial and venous circulation. This figure represents the means and standard errors for total nitrosylated hemoglobin in the arterial (solid line) and venous (dashed line) blood from five individuals before and during (solid bar on x axis) 80 ppm NO breathing. A trend toward an arterial venous gradient before (P = 0.09) and after (P = 0.11) NO breathing is observed. During NO breathing, a significant increase occurs in both the levels of arterial and venous nitrosylated hemoglobin (P < 0.001) and the arterial–venous gradient (P = 0.002).
The observation of a large arterial–venous gradient of total nitrosylated hemoglobin during NO breathing in normal volunteers and in patients with sickle cell anemia, with a plateau at 1–2 h of NO breathing, suggests delivery or rapid metabolism of NO in the microcirculation. If this NO were bound to the cysteine 93 of hemoglobin, this would constitute proof of delivery of NO from SNO-Hb. As mentioned previously, peripheral effects of inhaled NO have been advanced as evidence of delivery of NO from SNO-Hb (3, 12), and these results are consistent with such delivery. However, the specific binding site of NO on hemoglobin, the heme vs. cysteine 93, is undetermined.
Validation of a Sensitive and Specific Measurement of Hb(FeII)NO and SNO-Hb.
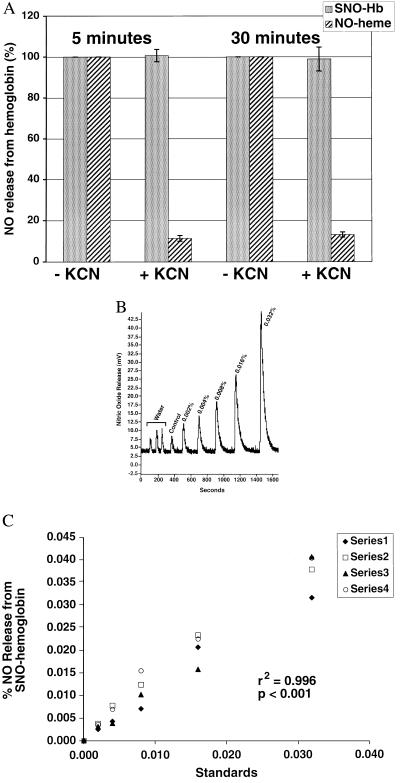
Therefore, we have developed a methodology to differentiate among the species of nitrosylated hemoglobin and to quantitate them in human arterial and venous blood at levels of 1 × 10−5 mol of NO per mol heme subunit. To establish the specific identity of the NO–hemoglobin adduct, pretreatment with and without a 100-fold molar excess of KCN and K3Fe(CN)6 was used to selectively remove the NO from heme while preserving the S-nitrosothiol bond. In fact, within 3 min, KCN/K3Fe(CN)6 (0.2 M) oxidizes the Fe-NO to an intermediate species not detectable in the chemiluminescent I3− reaction, while preserving the S–NO bond of SNO-Hb (Fig. 2A). The stability of the S-nitroso linkage in KCN/K3Fe(CN)6 has been tested for up to 2 h (n = 5) with less than 10% loss of NO signal.
Figure 2.
Sensitivity and specificity of I3−/ozone-based chemiluminescence assay, with and without KCN/K3Fe(CN)6, for the quantification of total nitrosylated hemoglobin, SNO-Hb, and Hb(FeII)NO. A demonstrates the reaction of standards of pure SNO-Hb (stippled bars) and Hb(FeII)NO (slashed bar) with and without KCN and K3Fe(CN)6 (indicated as + and −KCN, respectively), which selectively removes the NO from heme while preserving the S-nitrosothiol bond. Within 5 min, treatment with a large molar excess of KCN/K3Fe(CN)6 (0.2 M) eliminates 90% of the signal of Hb(FeII)NO in the chemiluminescent I3− reaction, while preserving the signal from SNO-Hb (n = 5). Results are expressed as the percentage of the original signal from the standards reacted without KCN/K3Fe(CN)6 pretreatment. Similar results were observed up to 120 min (data not shown). B demonstrates the NO signal released from three injections of water from the Sephadex G25 column (background control), followed by increasing standard dilutions of SNO-Hb, pretreated with KCN/K3Fe(CN)6 (control hemoglobin, 0.002%, 0.004%, 0.008%, 0.016%, 0.032%), measured by I3−/ozone-based chemiluminescence. C demonstrates measurement linearity of four serial dilutions of SNO-Hb, pretreated with KCN/K3Fe(CN)6, from 0.002% to 0.032% (r2 = 0.996; P < 0.001; n = 4). Similar results were obtained for standard curves of Hb(FeII)NO (r2 = 0.999, P < 0.001, n = 5).
The technique was validated by using pure synthesized species of SNO-Hb (specificity of β-cysteine 93 modification confirmed by HPLC electrospray mass spectrometry after enzymatic digestion) and Hb(FeII)NO (100% heme nitrosation confirmed by visible absorption spectroscopy). Synthesized standard preparations of SNO-Hb contained a mean 1.96 ± 0.17 mol of S-NO per mol hemoglobin tetramer (measurements performed by using SNO–glutathione standards), consistent with nitrosylation of the β-cysteine 93 residues on each β-chain. Standards of SNO-Hb and Hb(FeII)NO were diluted in fresh purified oxyhemoglobin to levels of 1 × 10−5 (0.001%) mol of NO per mol heme. We found linear sensitivity for total nitrosylated hemoglobin, SNO-Hb, and Hb(FeII)NO from 0.001% to 100% (SNO-Hb r2 = 0.996, P < 0.001, n = 5; Hb(FeII)NO r2 = 0.999, P < 0.001, n = 5). Four representative dilutions from 0.002% to 0.032% SNO-Hb are shown in Fig. 2 B and C. The assay is capable of measuring differences between control hemoglobin and 0.001% nitrosylated hemoglobin (n = 8, P = 0.007, data not shown).
Where Is the NO Bound?
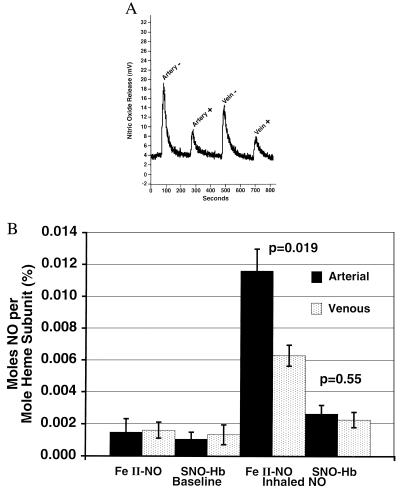
To determine whether the NO is transported as Hb(FeII)NO or SNO-Hb, we measured these two hemoglobin species in eight normal volunteers (five of the previously studied volunteers and three new), at baseline and during NO breathing. Importantly, in all samples, the predominant species formed was Hb(FeII)NO and not SNO-Hb (Fig. 3 A and B). Mean baseline levels of Hb(FeII)NO were 0.0015 ± 0.0008% in the arterial and 0.0016 ± 0.0005% in the venous circulation; during NO breathing, these values increased significantly to 0.012 ± 0.0014% and 0.006 ± 0.0007%, respectively, with a significant arterial–venous gradient developing (P = 0.019 for gradient). The arterial–venous difference suggests a very rapid dissociation rate of NO from heme and possible delivery of NO to the tissues.
Figure 3.
Basal and augmented levels of Hb(FeII)NO or SNO-Hb in eight normal volunteers measured with I3−/ozone-based chemiluminescence with and without KCN and K3Fe(CN)6 pretreatment, which selectively removes the NO from heme without affecting NO covalently bound to cysteine 93. A demonstrates the effects of KCN and K3Fe(CN)6 pretreatment on the signal of NO released from hemoglobin in I3− from a normal individual breathing 80 ppm NO for 2 h. KCN/K3Fe(CN)6 pretreatment is indicated by a + sign and no pretreatment by a − sign. A significant reduction in the NO signal after KCN and K3Fe(CN)6 pretreatment provides evidence that the majority of the NO associated with hemoglobin is bound to the heme group [Hb(FeII)NO]. The residual signal is still greater than baseline, suggesting that SNO-Hb does form at lower levels. Arterial (A)–venous (V) gradients are observed. B shows the means and standard errors for Hb(FeII)NO and SNO-Hb in the arterial (solid bars) and venous blood (stippled bars) from eight individuals at baseline and during 80 ppm NO breathing. Arterial-venous gradients in Hb(FeII)NO are significant (P = 0.019), whereas those of SNO-Hb are not (P = 0.55).
Mean baseline levels of SNO-Hb were 0.0011 ± 0.0004% in the arterial and 0.0013 ± 0.0006% in the venous circulation; during NO breathing, these values increased to 0.0026 ± 0.0005% (P = 0.11 for the increase) and 0.0022 ± 0.0005% (P = 0.3 for the increase), arterial and venous, respectively, without a significant arterial–venous gradient (P = 0.55 for gradient). The levels of SNO-Hb measured are not consistent with major levels of NO delivery by using this pathway in the human circulation, but they do provide evidence that this pathway exists.
Relative Production of FeIII, Hb(FeII)NO, and SNO-Hb.
Recently, Gow et al. have argued, from in vitro data, that the oxygen-induced conformational changes favor the formation of SNO-Hb over FeIII, and that under physiologic concentrations of phosphate (10 mM) and NO (1 μM), the production of SNO-Hb would be comparable to Hb(FeII)NO and would predominate over the formation of FeIII (12). In our in vivo study, FeIII levels were measured by using cooximetry (and validated by the method of Winterbourn) and revealed mean baseline levels of 0.24 ± 0.02% (22.8 μM heme), which increased to 0.9 ± 0.1% (84 μM heme) during NO breathing. Basal and NO breathing levels of FeIII were approximately 70-fold higher than Hb(FeII)NO levels (1.2 μM heme), which in turn were nearly 5-fold higher than the SNO-Hb levels (0.26 μM heme). Therefore, the in vivo reactions of NO with hemoglobin to form FeIII and nitrate >>> Hb(FeII)NO > SNO-Hb are consistent with the known kinetics of NO–hemoglobin interactions in vitro and the relative greater abundance of oxygenated compared with deoxygenated hemoglobin in the lung. The relative abundance of these species is consistent between basal and NO breathing states. It can be argued that NO breathing represents a supraphysiologic level of NO. Assuming a normal 5 liters/min ventilation and cardiac output, and if every molecule of NO diffuses into the bloodstream, the concentration (at standard temperature and pressure) would approach 4 μM. Although the ratio of NO to hemoglobin under these conditions is approximately 1:2,500, significantly less than the 1:1,000 ratio of NO to hemoglobin used in previous in vitro studies (12), it remains possible that the reactions at this concentration might be different from those of endogenously produced NO.
Plasma Nitrate, Nitrite, and Low Molecular Weight Nitrosothiol Levels Are Consistent with Significant NO–Heme Reactivity but Minimal Nitrosation: Considerations on the Origin of FeIII and Nitrate in the Human Circulation.
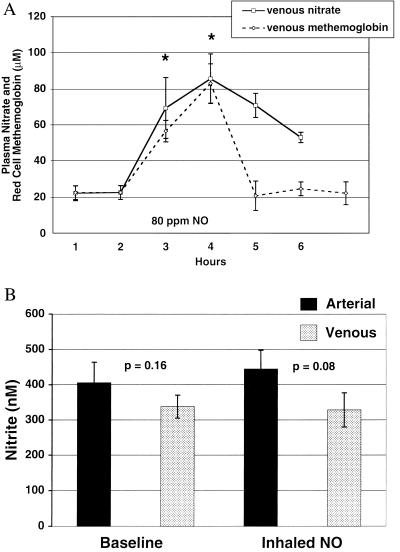
If the primary reaction of NO is with oxyhemoglobin, it would be expected that during NO inhalation significant levels of nitrate and FeIII would be generated at the alveolar–vascular interface. We measured venous plasma nitrate levels in five individuals by using chemiluminescence and found time-dependent increases during NO breathing (Fig. 4A) that correlate with increases in red cell FeIII concentration measured by cooximetry. It has recently been suggested that under physiological conditions, the primary reaction of NO with oxygenated erythrocytes (99% oxyhemoglobin) is to form Hb(FeII)NO, and that nitrate in the human circulation may not actually derive from NO–hemoglobin interactions (12). Our data suggest otherwise. At baseline and during NO inhalation, in fasting individuals, the micromolar FeIII concentrations of whole blood are almost identical to that of plasma nitrate. The persistent increased concentration of plasma nitrate after NO breathing likely represents the slower clearance of nitrate, which is eliminated unchanged in the urine, compared with FeIII, which is actively reduced by FeIII reductase. In a separate experiment in two other individuals, NO gas was administered at 80 ppm for 1 h, and plasma nitrate and FeIII were measured at 15-min intervals. The baseline levels and steady increases during NO inhalation correlated strongly with each other at all time points (r2 > 0.96, P < 0.001). These results suggest that nitrate and FeIII in the human blood likely derive primarily from the reaction of NO and oxyhemoglobin.
Figure 4.
Plasma nitrate and nitrite and FeIII levels at baseline and during 80 ppm NO breathing. A shows venous plasma nitrate (solid line) and whole blood FeIII (dashed line) levels measured for 2 h before NO inhalation, for 2 h during 80 ppm NO inhalation (x axis bar denotes NO inhalation), and for 2 h after NO inhalation. A significant increase, compared with baseline levels, in both plasma nitrate and erythrocyte FeIII is observed at 1 and 2 h of NO inhalation (*, P < 0.01 for both 1- and 2-h NO inhalation time points; n = 7). B shows arterial (solid bars) and venous (stippled bars) plasma nitrite levels from seven individuals at baseline and during 80 ppm NO inhalation. There is no significant increase in nitrite during NO breathing. P values are reported for arterial–venous differences.
Unlike nitrate, nitrite demonstrates no significant increase during NO breathing (n = 7; Fig. 4B). The lack of a significant increase in nitrite is consistent with the predominant reaction of NO gas at the alveolar–vascular interface being with oxyhemoglobin to form nitrate and FeIII. The reaction of NO and oxygen to form nitrosative intermediates (N2O3), which can nitrosate thiol groups and produce nitrite (25–28), may be limited by the low concentration of oxygen and NO dissolved in blood (even during NO breathing). This is further supported by our observations that at baseline and during NO breathing, arterial and venous levels of low molecular-weight nitrosothiols [measured by differential reaction in KI and I3− (20) and in Cu+/l-cysteine (22)] are unmeasurable and do not increase (data not shown, n = seven patients).
Discussion
It is evident that nitrosative pathways necessary to form nitrosothiols, nitrite, and SNO-Hb are limited in the human circulation, even during NO breathing. Rather, during NO breathing, NO predominately reacts with the heme moiety of hemoglobin either to form FeIII and nitrate or to form Hb(FeII)NO. This observation clearly establishes the dominant role of heme–NO reactions in vivo. The observation of a significant arterial–venous gradient in Hb(FeII)NO is unexpected for two reasons. First, assuming a circulatory time from the radial artery to the antecubital vein of less than 30 s, the observed 50% reduction in Hb(FeII)NO from artery to vein represents a very rapid in vivo NO dissociation rate, much greater than that measured in vitro. Second, it is generally believed that Hb(FeII)NO is formed by the reaction of NO with deoxygenated hemoglobin. Although these results show that physiologically FeIII formation far exceeds the rate of Hb(FeII)NO formation, NO could occupy deoxygenated heme groups on partially oxygenated hemoglobin, possibly successfully competing with oxygen for these sites. This observation is consistent with recent work highlighting the significance of heme–NO interactions under physiological conditions (12). These new data shift the focus of NO transport on hemoglobin from β-cysteine 93 to the heme groups and raise questions about the mechanisms of NO release, the nature of the NO metabolite released, the physiological effects of this process on oxygen delivery, and blood flow.
NO has a very high affinity for the heme moiety of deoxyhemoglobin with a Ka of 2 × 107 M−1⋅s−1 and Kd of 3 × 10−3⋅s−1 (29). This affinity is greater than for either oxygen or carbon monoxide, with a half life of 16 h at 15°C (for tetranitrosylhemoglobin) and 41 min at 37°C (7). Even at this higher rate of dissociation, it would be expected that inhaled NO at 80 ppm would result in steady accumulation of Hb(FeII)NO, ultimately interfering with oxygen transport. Similar to carbon monoxide and cyanide poisoning, the high Ka and low Kd of NO for hemoglobin should result in NO poisoning, particularly during NO inhalation and septic shock (a disease characterized by increased inducible nitric oxide synthase expression). Our observation of a very fast decomposition rate helps explain this lack of NO–heme accumulation. There is precedent for a rapid dissociation of NO from a heme moiety; a half life of 21 min has been noted for intact erythrocytes at 37°C (30). In vitro kinetic studies of NO dissociation from the heme of soluble guanylate cyclase in the presence of GTP and Mg2+ reveal a half life of approximately 5 s (31). It is likely that the red cell possesses unique mechanisms to facilitate the dissociation of NO from hemoglobin. Although our in vitro studies have suggested that NO gas reacts primarily with the heme groups of hemoglobin, it deserves mention that it is also possible that some of the NO signal measured in vivo represents binding to a site other than the heme group, such as the relatively hydrophobic xenon-binding cavities elucidated by Schoenborn (32).
The end result of this dissociation is of significant interest. It has been theorized that Hb(FeII)NO reacts with oxygen, forming a heme–peroxynitrite adduct and finally liberating nitrate (29). It is possible that nitrite and NO may also be released as evidenced by the reaction of Hb(FeII)NO in I3−, resulting in stoichiometric release of NO gas. K3FeIII(CN)6 treatment of Hb(FeII)NO in the absence of cyanide results in NO release from heme in a form measurable in I3−, suggesting release as NO+ or nitrite (data not shown). FeIII [present in 100-fold excess of Hb(FeII)NO during NO breathing], superoxide, or other sources of iron or other metals, could have a similar effect. NO can be redox activated by FeIII porphyrins and heme proteins to a nitrosating species, likely NO+ or nitrite (33, 34). In the red cell, the concentration of glutathione is 5 mM, whereas that of β-cysteine 93 is approximately 10 mM (based on a 5-mM hemoglobin concentration). It is therefore possible that FeIII redox activated NO (NO+) could react with glutathione, undergo an intra- or interhemoglobin transfer to cysteine 93, or react with water to form nitrite. Nitrite under physiologic acidity (pH < 7) forms nitrous acid with spontaneous decomposition to NO (35, 36), providing a further source of NO in the peripheral vasculature.
The conserved nature of hemoglobin β-cysteine 93 in all mammals and most vertebrates (37), despite the fact that it is not sterically crucial (alanine substitution does not affect the hemoglobin tertiary structure), has been a mystery to hemoglobin biologists. The model of NO delivery on β-cysteine 93 appeared to have solved this mystery (1–3). However, whereas our results demonstrate the existence of a SNO-Hb pathway in humans, NO–heme reactions appear to predominate. Furthermore, the low levels of SNO-Hb and lack of a significant arterial–venous gradient, even during NO breathing, suggest that minimal NO is delivered by this pathway under basal conditions. In light of these apparently contradictory data about the role of cysteine 93 in NO delivery, it is necessary to address alternative explanations for the invariant presence of cysteine 93 in mammalian hemoglobins.
One might postulate three functions for cysteine 93: (i) to facilitate the unloading of NO from heme; (ii) to facilitate the delivery of NO specifically to hypoxic tissues; and (iii) to serve as an electron acceptor or donor contributing to heme redox equilibrium. First, as discussed above, it is possible that cysteine 93 evolved as an acceptor of NO released from Hb(FeII)NO. NO+ release from heme could undergo geminate recombination with intramolecular cysteine 93 or intermolecular transfer to a neighboring hemoglobin molecule. The NO could then be transported out of the erythrocyte by transnitrosation with glutathione. This model is consistent with data generated by Stamler and colleagues (1–3), but it invokes a primary reaction with heme groups. Second, all of our measurements were made in the circulation of the resting arm. It remains possible that across other vascular beds (organ specific or ischemic), the extraction rate of NO may increase, creating an arterial–venous gradient in SNO-Hb. Recent studies demonstrate that SNO-Hb has an increased oxygen affinity with intact Bohr and phosphate effects, suggesting that it would deliver only its oxygen and thus release its NO to regions with low pH and/or very low oxygen tension (4, 5). Thus, hemoglobin S-nitrosation may be a “salvage” pathway, providing the red cell with a mechanism to deliver NO only in regions with significant stress (reduced blood flow, tissue hypoxia, and acidosis) where hemoglobin-NO scavenging would be deleterious.
A third potential function for cysteine 93 could be a role as an electron donor/acceptor from the β-globin heme necessary for heme redox equilibrium, as originally suggested by Winterbourn and Carrell (24). [The distance from the iron to the sulfur is 12 Å, and the distance from the center of the proximal histidine to the sulfur is 10 Å. Electron transfer over such distances has been found to occur in many proteins (M. F. Perutz, personal communication).] They demonstrated that β-chain heme oxidation by Cu(II) required binding to cysteine 93. This observation has been supported by Rifkind and colleagues, who showed by low-temperature electron paramagnetic resonance spectroscopy that heme autooxidation occurs with the formation of superoxide in the heme pocket (38). Simultaneously, a thiyl radical forms that can be blocked by N-ethylmaleimide, suggesting that reversible electron transfer between the cysteine 93 residue and superoxide in the β-heme pocket occurs. Bonaventura and colleagues also demonstrated that Cu(II) accelerates the observed reduction of metHb by CO/H2O by a factor of 1,000, and that this effect of Cu(II) is largely eliminated by blocking cysteine 93 (39). They hypothesized that Cu(II) binding to cysteine 93 results in electron movement between the globin chain and the heme group. These studies provide evidence that electron movement through the β-globin occurs between cysteine and heme and may contribute to FeIII reduction. These same mechanisms could facilitate NO release from heme as a delivery or detoxification pathway.
In conclusion, our results demonstrate that NO–heme reaction pathways predominate in vivo and suggest that NO binding to heme groups is a rapidly reversible process, supporting a revised model of hemoglobin delivery of NO in the peripheral circulation. Direct NO, NO+, or nitrite release from Hb(FeII)NO, with SNO-Hb possibly serving as an intermediary, may explain the peripheral vascular effects reported during NO breathing and may represent a salvage pathway for maintenance of vascular flow in regions of tissue stress; such pathways could be fertile areas for pharmacological interventions.
Acknowledgments
We thank Drs. Robert Danner, Anthony Suffredini, Celia Bonaventura, and Connie Noguchi for very valuable discussions and suggestions, Drs. Larry Keefer, David Wink, and Jay Zweier for providing their expertise with NO chemistry, and the Critical Care Medicine Department respiratory therapy section for assistance with NO delivery and blood gas analysis.
Abbreviations
- NO
nitric oxide
- SNO-Hb
S-nitrosohemoglobin
- Hb(FeII)NO
nitrosyl(heme)hemoglobin
- FeIII
methemoglobin
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.180155397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.180155397
References
- 1.Jia L, Bonaventura C, Bonaventura J, Stamler J S. Nature (London) 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 2.Stamler J S, Jia L, Eu J P, McMahon T J, Demchenko I T, Bonaventura J, Gernert K, Piantadosi C A. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 3.Gow A J, Stamler J S. Nature (London) 1998;391:169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- 4.Bonaventura C, Ferruzzi G, Tesh S, Stevens R D. J Biol Chem. 1999;274:24742–24748. doi: 10.1074/jbc.274.35.24742. [DOI] [PubMed] [Google Scholar]
- 5.Patel R P, Hogg N, Spencer N Y, Kalyanaraman B, Matalon S, Darley-Usmar V M. J Biol Chem. 1999;274:15487–15492. doi: 10.1074/jbc.274.22.15487. [DOI] [PubMed] [Google Scholar]
- 6.Wolzt M, MacAllister R J, Davis D, Feelisch M, Moncada S, Vallance P, Hobbs A J. J Biol Chem. 1999;274:28983–28990. doi: 10.1074/jbc.274.41.28983. [DOI] [PubMed] [Google Scholar]
- 7.Yonetani T, Tsuneshige A, Zhou Y, Chen X. J Biol Chem. 1998;273:20323–20333. doi: 10.1074/jbc.273.32.20323. [DOI] [PubMed] [Google Scholar]
- 8.Kosaka H, Sawai Y, Sakaguchi H, Kumura E, Harada N, Watanabe M, Shiga T. Am J Physiol. 1994;266:C1400–C1405. doi: 10.1152/ajpcell.1994.266.5.C1400. [DOI] [PubMed] [Google Scholar]
- 9.Kosaka H, Seiyama A. Biochem Biophys Res Commun. 1996;218:749–752. doi: 10.1006/bbrc.1996.0133. [DOI] [PubMed] [Google Scholar]
- 10.Kosaka H, Seiyama A. Nat Med. 1997;3:456–459. doi: 10.1038/nm0497-456. [DOI] [PubMed] [Google Scholar]
- 11.Kosaka H. Biochim Biophys Acta. 1999;1411:370–377. doi: 10.1016/s0005-2728(99)00026-2. [DOI] [PubMed] [Google Scholar]
- 12.Gow A J, Luchsinger B P, Pawloski J R, Singel D J, Stamler J S. Proc Natl Acad Sci USA. 1999;96:9027–9032. doi: 10.1073/pnas.96.16.9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross S S, Lane P. Proc Natl Acad Sci USA. 1999;96:9967–9969. doi: 10.1073/pnas.96.18.9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi Y, Kobayashi H, Tanaka N, Sato T, Takizawa N, Tomita T. Am J Physiol. 1998;274:H349–H357. doi: 10.1152/ajpheart.1998.274.1.H349. [DOI] [PubMed] [Google Scholar]
- 15.Quezado Z M, Natanson C, Karzai W, Danner R L, Koev C A, Fitz Y, Dolan D P, Richmond S, Banks S M, Wilson L, Eichacker P Q. J Appl Physiol. 1998;84:107–115. doi: 10.1152/jappl.1998.84.1.107. [DOI] [PubMed] [Google Scholar]
- 16.Troncy E, Francoeur M, Salazkin I, Yang F, Charbonneau M, Leclerc G, Vinay P, Blaise G. Br J Anaesth. 1997;79:631–640. doi: 10.1093/bja/79.5.631. [DOI] [PubMed] [Google Scholar]
- 17.Fox-Robichaud A, Payne D, Hasan S U, Ostrovsky L, Fairhead T, Reinhardt P, Kubes P. J Clin Invest. 1998;101:2497–2505. doi: 10.1172/JCI2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubes P, Payne D, Grisham M B, Jourd-Heuil D, Fox-Robichaud A. Am J Physiol. 1999;277:H676–H682. doi: 10.1152/ajpheart.1999.277.2.H676. [DOI] [PubMed] [Google Scholar]
- 19.Kermarrec N, Zunic P, Beloucif S, Benessiano J, Drouet L, Payen D. Am J Respir Crit Care Med. 1998;158:833–839. doi: 10.1164/ajrccm.158.3.9709097. [DOI] [PubMed] [Google Scholar]
- 20.Samouilov A, Zweier J L. Anal Biochem. 1998;258:322–330. doi: 10.1006/abio.1998.2609. [DOI] [PubMed] [Google Scholar]
- 21.Gladwin M T, Schechter A N, Shelhamer J H, Pannell L K, Conway D A, Hrinczenko B W, Nichols J S, Pease-Fye M E, Noguchi C T, Rodgers G P, Ognibene F P. J Clin Invest. 1999;104:937–945. doi: 10.1172/JCI7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang K, Ragsdale N V, Carey R M, MacDonald T, Gaston B. Biochem Biophys Res Commun. 1998;252:535–540. doi: 10.1006/bbrc.1998.9688. [DOI] [PubMed] [Google Scholar]
- 23.Ewing J F, Janero D R. Free Rad Biol Med. 1998;25:621–628. doi: 10.1016/s0891-5849(98)00083-5. [DOI] [PubMed] [Google Scholar]
- 24.Winterbourn C C, Carrell R W. Biochem J. 1977;165:141–148. doi: 10.1042/bj1650141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wink D A, Mitchell J B. Free Radic Biol Med. 1998;25:434–456. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 26.Wink D A, Darbyshire J F, Nims R W, Saavedra J E, Ford P C. Chem Res Toxicol. 1993;6:23–27. doi: 10.1021/tx00031a003. [DOI] [PubMed] [Google Scholar]
- 27.Wink D A, Nims R W, Darbyshire J F, Christodoulou D, Hanbauer I, Cox G W, Laval F, Laval J, Cook J A, Krishna M C, et al. Chem Res Toxicol. 1994;7:519–525. doi: 10.1021/tx00040a007. [DOI] [PubMed] [Google Scholar]
- 28.Kharitonov V G, Sundquist A R, Sharma V S. J Biol Chem. 1995;270:28158–28164. doi: 10.1074/jbc.270.47.28158. [DOI] [PubMed] [Google Scholar]
- 29.Cooper C E. Biochim Biophys Acta. 1999;1411:290–309. doi: 10.1016/s0005-2728(99)00021-3. [DOI] [PubMed] [Google Scholar]
- 30.Huang T H. J Biol Chem. 1979;254:11467–11474. [PubMed] [Google Scholar]
- 31.Kharitonov V G, Russwurm M, Magde D, Sharma V S, Koesling D. Biochem Biophys Res Commun. 1997;239:284–286. doi: 10.1006/bbrc.1997.7470. [DOI] [PubMed] [Google Scholar]
- 32.Schoenborn B P. Nature (London) 1965;208:760–762. doi: 10.1038/208760a0. [DOI] [PubMed] [Google Scholar]
- 33.Wade R S, Castro C E. Chem Res Toxicol. 1990;3:289–291. doi: 10.1021/tx00016a002. [DOI] [PubMed] [Google Scholar]
- 34.Stubauer G, Giuffre A, Sarti P. J Biol Chem. 1999;274:28128–28133. doi: 10.1074/jbc.274.40.28128. [DOI] [PubMed] [Google Scholar]
- 35.Zweier J L, Wang P, Samouilov A, Kuppusamy P. Nat Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 36.Zweier J L, Samouilov A, Kuppusamy P. Biochim Biophys Acta. 1999;1411:250–262. doi: 10.1016/s0005-2728(99)00018-3. [DOI] [PubMed] [Google Scholar]
- 37.Perutz M F. Nature (London) 1996;380:205–206. doi: 10.1038/380205b0. [DOI] [PubMed] [Google Scholar]
- 38.Balagopalakrishna C, Abugo O O, Horsky J, Manoharan P T, Nagababu E, Rifkind J M. Biochemistry. 1998;37:13194–13202. doi: 10.1021/bi980941c. [DOI] [PubMed] [Google Scholar]
- 39.Bonaventura C, Godette G, Tesh S, Holm D E, Bonaventura J, Crumbliss A L, Pearce L L, Peterson J. J Biol Chem. 1999;274:5499–5507. doi: 10.1074/jbc.274.9.5499. [DOI] [PubMed] [Google Scholar]