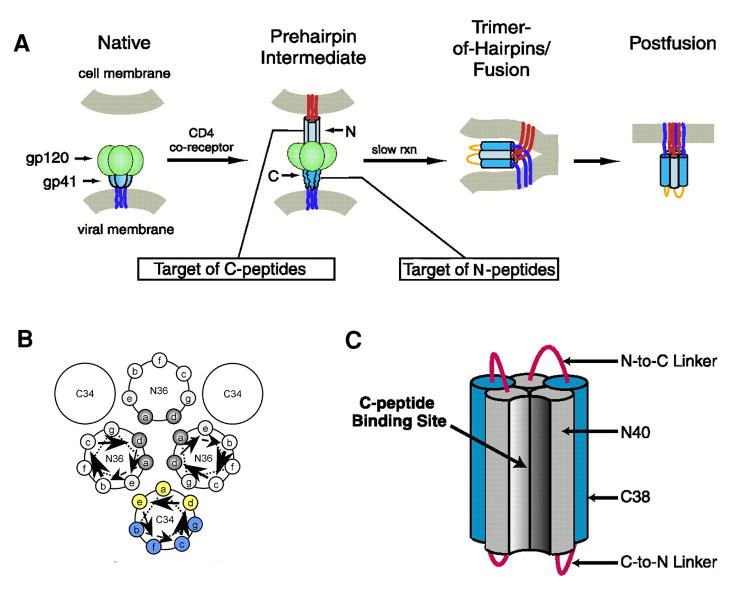
Figure 3.
Mechanism of action of HIV-1 entry inhibitors. Panel A illustrates the involvement of gp41 in the fusion of the cell and viral membranes and the interference of peptide fusion inhibitors with this event. The inhibitors are believed to prevent formation of the 6-helix hairpin bundle necessary for juxtaposing the cell and viral membranes. C-HR peptides like T-20 bind to the N-HR trimeric core and N-peptides form a trimer which binds to the C-HR helix forming heterocomplexes that are defective in fusion. Panel B Schematic helical wheel diagram used in the design of C-peptides with increased helicity and water solubility. The positions that pack against the N-trimeric core (indicated in yellow) are less favored for replacement than those facing away (indicated in blue). Panel C. 5- Helix inhibitor. The hetero bundle lacks one C-peptide and thus provides a binding pocket for the C-HR peptide of gp41. Adapted from [13,38].