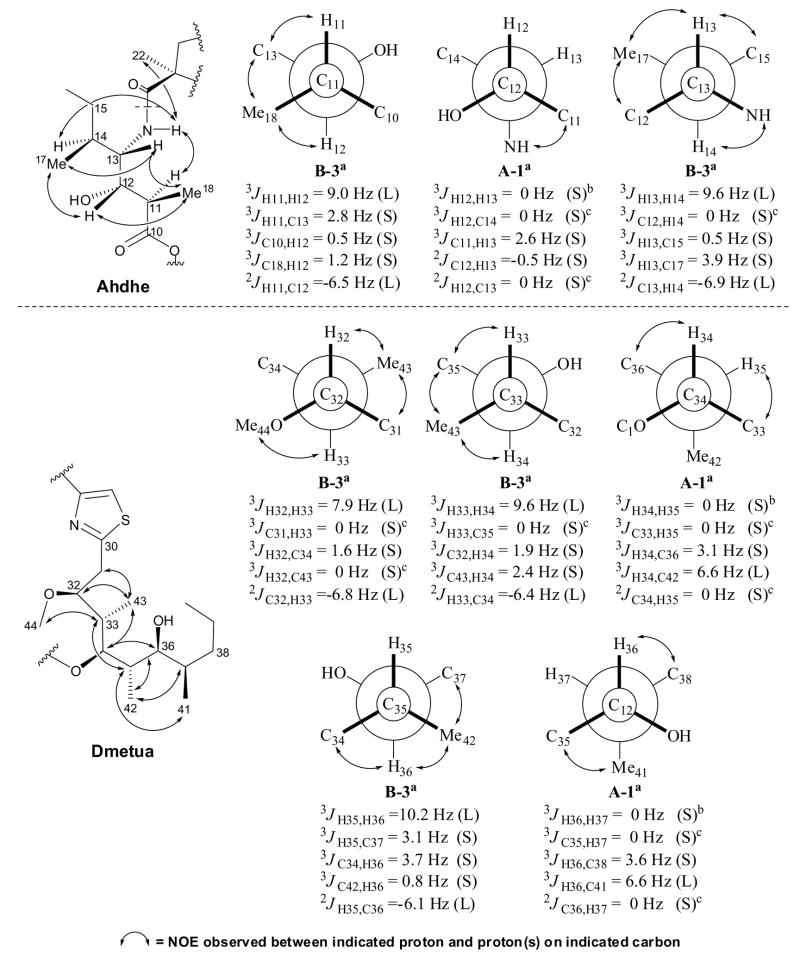
Figure 2.
Homonuclear and heteronuclear coupling constant values used to assign the relative configuration of Ahdhe (C10-C18) and Dmetua (C30-C44). The magnitude of the coupling constants, (S) for small and (L) for large, allowed identification of gauche and anti orientations between the indicated atoms. For 1,2-methine systems in which the threo rotamer A3 can not be distinguished from the erythro rotamer B3, NOE data (↔) provided an unambiguous assignment. aRelative configuration and rotamer designation according to Matsumori et al. (1999). bWeak couplings with 3JH,H < 0.5 Hz were considered as 0 Hz. cNo correlation observed in HSQMBC as expected for a coupling of 0 Hz.