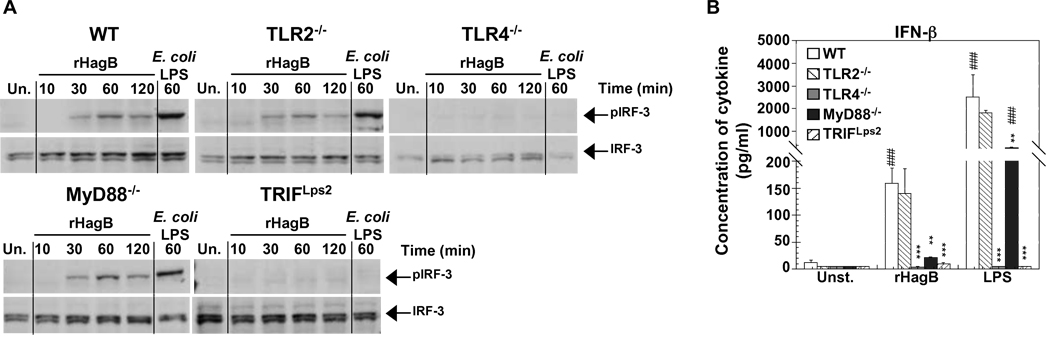
Fig. 8.
Phosphorylation of IRF-3 and production of IFN-β by rHagB stimulated DC is dependent on TLR4 signaling and the adaptor molecule TRIF. (A) DC from WT, TLR2−/−, TLR4−/−, MyD88−/− and TRIF Lps2 mice were stimulated with 40 µg/ml rHagB for 10, 30, 60 or 120 min. Following stimulation, cells were lysed and whole cell lysates were tested for phosphorylation of IRF-3 by Western blot. Total IRF-3 was used as a loading control. Unstimulated DC (far left lanes) or DC stimulated with 100 ng/ml E. coli K12 LPS for 60 min (far right lanes) were used as controls. Results are representative of two independent experiments. (B) DC (2×105) were stimulated with 40 µg/ml rHagB, 100 ng/ml of E. coli K12 LPS or left untreated. Culture supernatants were harvested 24 h post-stimulation and assayed for IFN-β production by ELISA. Results are expressed as the mean ± standard error of duplicate cultures from three independent experiments. *** and ** Significant differences at P < 0.001 and P < 0.01, respectively, compared to WT cultures stimulated with rHagB or LPS. ### Significant differences at P < 0.001 compared to control unstimulated cultures.