Abstract
Objective
To compare the tensile biomechanical properties of age-matched adult human articular cartilage from normal, degenerate, and osteoarthritic knees, and to determine the relationships between tensile properties and biochemical and structural properties hypothesized to underlie functional biomechanical deterioration.
Methods
Age-matched articular cartilage samples were obtained from the lateral and medial femoral condyles (LFC and MFC) of knees that exhibited (1) minimal fibrillation, characteristic of normal aging (NLA), (2) overt fibrillation associated with degeneration (DGN), or (3) overt fibrillation associated with osteoarthritis (OA). DGN samples were from knees that exhibited degeneration but not osteophytes while OA samples were from fragments removed during total knee arthroplasty. Cartilage samples were analyzed for tensile properties, cell and matrix composition, and histopathological structure.
Results
Differences in tensile, compositional, and surface structural properties were indicative of distinct stages of cartilage degeneration, early, advanced, and late, with early degenerative changes in NLA samples being more advanced in the MFC than the LFC, including higher surface fibrillation, lower intrinsic fluorescence, and lower mechanical integrity. The transition from early to advanced degeneration involved a diminution in mechanical function, surface integrity, and intrinsic fluorescence. The transition from advanced to late degeneration involved an increase in cartilage water content, an increase in degraded collagen, and loss of collagen.
Conclusions
These results provide evidence of coordinated mechanical dysfunction, collagen network remodeling, and surface fibrillation. Even in the cartilage of knees exhibiting overt fibrillation but not extensive erosions characteristic of clinical OA, most features of advanced cartilage degeneration were present.
Keywords: cartilage degeneration, tensile properties, wear, fluorescence, human articular cartilage
INTRODUCTION
Aging, cartilage fibrillation, and osteoarthritis (OA) may be contributing factors to the site-specific deterioration of the biomechanical function of articular cartilage in the knee joint. The effects of these factors can be difficult to separate because the incidence of both cartilage fibrillation and clinical OA increases with aging1. The tensile biomechanical properties of human knee articular cartilage vary between macroscopically normal,2–4 fibrillated normal, and fibrillated OA statees4 due to pathological state, age and site within the knee (Table 1 and supplement). Normal aging is associated with marked decreases in tensile modulus and strength in the LFC, but not in the MFC where tensile properties were already low in young adults.
Table 1.
Summary of studies of adult human knee articular cartilage examining association between tensile properties of cartilage surface layer with cartilage degeneration and joint osteoarthritis, age, and site.
| Tensile Properties of Surface Layer (MPa) | ||||||
|---|---|---|---|---|---|---|
| Reference | Condition of Joint or Articular Cartilage | Site | # Knees | Age (yrs) | Strength | Modulus |
| Kempson (1982)2 | Normal (Indian Ink)15 | Averaged for LFC and MFC | 5 | ~24–40 | ~35 | |
| 3 | ~41–60 | ~20 | ||||
| 7 | ~61–80 | ~15 | ||||
| Akizuki + (1986)4 | Normal (Indian Ink) | MFC in low weight bearing area | 4 | 24–42 | 10.1 | |
| Partial fibrillation | 2 | 52, 60 | 8.5 | |||
| Osteoarthritic | 3 | 65, 68, 74 | 1.4 | |||
| Temple + (2007)3 | Normal (Indian Ink & Histopathology) | Anterior LFC | 9 | 21–39 | 19 | 24 |
| 9 | 40–59 | 12 | 19 | |||
| 10 | 60–91 | 11 | 17 | |||
| Normal (Indian Ink & Histopathology) | Anterior MFC | 9 | 21–39 | 9 | 11 | |
| 10 | 40–59 | 8 | 11 | |||
| 9 | 60–91 | 10 | 12 | |||
Biomechanical failure of articular cartilage in degeneration and in OA has been hypothesized to be due to (1) degradation and loss of collagen and proteoglycan matrix components, (2) abnormal collagen network remodeling, (3) consequences of decreased cellularity, and (4) mechanical wear. The tensile stiffness and strength of cartilage depend on the organization of the collagen network, with highest values normally at the articular surface where collagen fibers are aligned along the tangential axis of testing5,6. Fragmentation and loss of collagen molecules are increased at sites adjacent to focal cartilage lesions7. Collagen degradation reduces the tensile stiffness and strength of articular cartilage,8 whereas proteoglycan extraction reduces compressive9 but not tensile stiffness10. Together, these results suggest that collagen degradation and loss contribute primarily to the tensile biomechanical weakening of human cartilage.
Abnormal collagen network remodeling, comprised of both synthesis and degradation of collagen, has also been postulated to result in cartilage weakening. With the upregulated synthesis of matrix molecules including collagen in OA cartilage11, collagen content can be maintained despite collagen degradation and be manifest as diminished intrinsic cartilage fluorescence in clinical OA12 and experimental animal models13. This rapidly-metabolized collagen may result in a network that has a reduced ability to withstand tensile loads in early cartilage degeneration.
Alteration in the cellular content of cartilage has also been implicated in cartilage weakening associated with cartilage degeneration and development of OA. The cell density of adult articular cartilage is decreased with cartilage fibrillation14. Such decreased cell density may be detrimental to matrix homeostasis and lead to tissue deterioration and, thus, cartilage biomechanical function.
Finally, mechanical wear could directly cause cartilage weakening at the articular surface. India ink staining highlights alteration of the articular cartilage surface which is slight with normal aging3. With severe wear and erosion of the cartilage surface that are characteristic of OA, India ink staining of the articular surface is considerable15.
Thus, these proposed mechanisms of biomechanical weakening may, individually or in concert, contribute to the deterioration of cartilage biomechanical function and the progression of OA disease. The hypothesis of this study was that aged human articular cartilage exhibits tensile weakening that is associated with variations in tissue composition and structure, in a depth- and site-associated manner, indicative of one or more of the postulated mechanisms of cartilage deterioration. The specific aims of this study were to characterize and compare articular cartilage from NLA, DGN, and OA joints, isolated from different depths at the LFC and MFC sites in terms of (1) tensile biomechanical properties, (2) density of cells, (3) content of extracellular matrix components, and (4) structure of the articular surface. By examining NLA, DGN, and OA samples, the results of this study were interpreted in terms of stages of cartilage degeneration.
MATERIALS AND METHODS
Sample Selection and Preparation
Age-matched samples (mean±SEM, 68±2 yrs, range 50–91 yrs, Table 2) in the form of 10-mm osteochondral cores were isolated using a surgical instrument (Osteochondral Autograft Transfer System; Arthrex, Naples, FL) from the anterior region of the MFC (n=24 cores) and LFC (n=24 cores) approximately 1.5cm lateral or medial to the intercondylar notch. The accuracy of the core position within a knee was ~0.5cm. The cores displayed (1) mild age-associated surface roughening of the articular cartilage surface, grade 1 as described in 16 (NLA, n=23 cores from 14 donors), (2) overt fibrillation, grade 3 16 associated with degeneration but not OA (DGN, n=12 cores from 8 donors), or overt fibrillation, also grade 3 16, but associated with OA (OA, n=13 cores from 11 donors). NLA and DGN samples were from twenty-two cadavers obtained from tissue banks with donation areas in the Western and Southern areas of the United States. Cadaveric knee joints were stored at 4°C prior to shipment, shipped on wet ice, and obtained within 48hrs of death. OA samples were obtained with Institutional Review Board approval from eleven patients undergoing TKR, stored at 4°C, and obtained within 16hrs. In all, samples were from one knee of each of 33 donors, from both the LFC and MFC of most (9/14) NLA knees and many (4/8) DGN knees, but relatively few (2/11) OA knees.
Table 2.
Sample, joint, and donor characteristics. Age, macroscopic grades16 (grade 1: normal, intact surface; grade 2: minimal fibrillation; grade 3: overt fibrillation; grade 4: full thickness erosion) of samples from the medial and lateral femoral condyles and overall joint, percentage area of full thickness cartilage erosion of the femoral condyles and joint, presence of osteophytes, body mass index (BMI), and n of human female and male donors of osteochondral cores from the LFC and MFC. Data are reported as mean ± SEM.
| Lateral Femoral Condyle | Medial Femoral Condyle | |||||
|---|---|---|---|---|---|---|
| NLA | DGN | OA | NLA | DGN | OA | |
| Age (yr) | 68±3 | 68±6 | 69±5 | 67±3 | 69±5 | 68±3 |
| Age range (yr) | 50–91 | 52–87 | 52–82 | 50–78 | 52–84 | 59–82 |
| Sample grade | 1 | 3 | 3 | 1 | 3 | 3 |
| Joint grade | 3.0±0.2 | 4±0 | 4±0 | 2.9±0.2 | 3.8±0.2 | 4.0±0.0 |
| Area of erosion, FC (%) | 0±0 | 6±4* | 22±3***, ††† | 0±0 | 6±4* | 21±4***, ††† |
| Area of erosion, Joint (%) | 0.1±0.1 | 15±4*** | 38±7***, ††† | 0±0 | 11±5* | 31±8***, † |
| Presence of osteophytes | 0/13 | 0/6 | 5/6 | 0/11 | 0/6 | 6/7 |
| BMI (kg/m2) | 24±2 | 27±5 | 30±1 | 22±2 | 25±3 | 28±1 |
| Female | 5 | 4 | 5 | 5 | 3 | 5 |
| Male | 7 | 2 | 1 | 6 | 3 | 2 |
| Female + male | 12 | 6 | 6 | 11 | 6 | 7 |
p<0.05,
p<0.005 versus NLA samples.
p<0.05,
p<0.005 versus DGN samples.
While the fibrillated cartilage surfaces of DGN and OA samples appeared grossly similar, the above criteria clearly distinguished the status of the knee joints, with the OA knee joints having cartilage degeneration and erosion that was much more extensive overall than DGN knee joints. The extent of cartilage erosion and OA disease was characterized by the overall joint grade and presence of osteophytes (Table 2) and further quantified as the area of full thickness cartilage erosion (as measured from digitized gross images of the joint surfaces, Table 2). Because of the presence of osteophytes in most joints from which OA samples were obtained, and because the area of cartilage erosion on the femoral condyles and the joint overall was higher in OA samples than DGN or NL samples (supplement), these experimental groups were considered to represent distinct stages of cartilage degeneration.
Tissue samples were graded macroscopically16, isolated, and immersed in phosphate buffered saline with proteinase inhibitors (PBS with PI)17 at 4°C for 1hr, and then stored at −70°C until the time of testing. Samples were thawed in ~1ml of PBS with PI for 15min at room temperature prior to analysis. Previous studies indicate that cartilage mechanical properties are not affected by a single freeze-thaw cycle18.
Structural Indices of Fibrillation
Samples were analyzed for cartilage thickness and surface roughness (reflectance score after India ink staining) as described previously3,17. An osteochondral fragment was isolated for histopathological analysis (Mankin-Shapiro score including surface irregularity) from a region adjacent to cartilage used for biomechanical and biochemical analyses17.
Biomechanical Properties
The remaining cartilage of each core was sliced into ~0.3-mm thick layers, at a distance from the articular surface of 0% (superficial layer, including the articular surface), 30% (middle layer), and 60% (deep layer) of the average cartilage thickness. A portion of the slices were cut into tapered specimens with the gage region oriented in the medial-lateral direction, parallel to the splitline direction typical for this site5 for equilibrium and constant strain-rate tensile testing, which was performed as described previously3,19.
Biochemical Properties
The remainders of the tissue slices, adjacent to tensile samples, were analyzed for cell and matrix components. A portion was weighed wet, lyophilized, weighed dry, solubilized with proteinase K and analyzed for DNA20, hydroxyproline21, intrinsic fluorescence20, and sulfated glycosaminoglycan (GAG)22. The remaining portions were analyzed for degraded collagen (COL in αCT) by analyzing the guanidine extracted cartilage for alpha-chymotrypsin extractable collagen3,23. DNA was converted to cell number assuming 7.3 pg DNA/human chondrocyte24. Hydroxyproline content was converted to collagen (COL) content using a mass ratio of collagen to hydroxyproline of 7.125. Intrinsic fluorescence was measured at excitation (Ex) and emission (Em) wavelengths corresponding to the maximum fluorescence of pyridinoline (Ex 295/Em 395 nm) and pentosidine (Ex 335/Em 385 nm) crosslinks, and the intrinsic fluorescence was reported as a ratio of pentosidine-associated to pyridinoline-associated fluorescence (fluorescence ratio)3. The contents of DNA, COL, and GAG were calculated as the mass normalized to wet weight.
Statistics
Initially, the effect of degeneration on the various mechanical and biochemical parameters was assessed using repeated measures ANOVA with anatomical location (LFC or MFC) and depth from the surface (superficial, middle, or deep) as repeated factors. Then, for all variables analyzed, the effects of degeneration and depth were analyzed for each anatomical location, LFC and MFC, separately. When experimental group or depth from the articular surface had an effect, planned comparisons were made between experimental groups at each depth. The effects of depth within each experimental group were not analyzed to maintain statistical power. To limit the experiment-wise error rate, each comparison was tested using a significance level alpha = 0.05/number of comparisons26. Percentage data were arcsine transformed to improve normality prior to statistical analyses. For ordinal data (i.e., histopathology index and surface irregularity), the effects of location and experimental group were tested using the Scheirer-Ray-Hare test26, followed by a Dunn’s test for specific group comparisons. All data are reported as mean ± SEM.
Relationships between mechanical parameters and age, structural parameters, and biochemical parameters were assessed by parametric univariate linear regression analysis as well as multivariate linear regression using the backward elimination procedure. The relationship between each mechanical parameter and histopathology index was assessed by the nonparametric Spearman’s rank method. To determine the ability of the structural, biochemical, and biomechanical indices to distinguish between the macroscopically normal and degenerate samples, a receiver operating characteristic curve was generated and analyzed as previously described17, with methods and results detailed in the supplement.
RESULTS
Structural Indices of Fibrillation
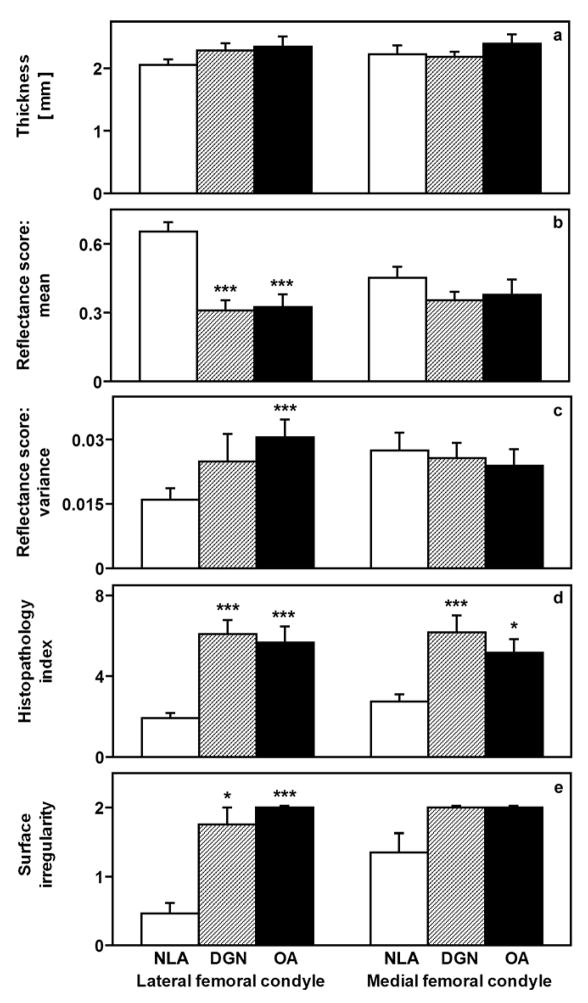
Indices of cartilage fibrillation indicated that the articular surfaces of NLA cartilage samples were mildly roughened while those of DGN and OA samples were overtly fibrillated. Overall cartilage thickness (Figure 1a) was similar for NLA, DGN, and OA groups (p=0.6) and for LFC and MFC sites (p=0.6). Overt cartilage fibrillation was evident as more intense ink-staining (lower reflectance score, Figure 1b) and higher variance of the reflectance score (an index of the surface roughness, Figure 1c) in DGN and OA than NLA samples (supplement). Differences in the reflectance score between groups varied inversely with the histopathology index (Figure 1d), whose values indicated advanced cartilage degeneration, and the surface irregularity histopathology score (Figure 1e). Thus, NLA samples exhibited surface roughening, while DGN and OA samples exhibited severe cartilage degeneration with overt fibrillation but had cartilage thickness similar to that of NLA samples.
Figure 1.
Structural and surface properties of human articular cartilage from the LFC and MFC. Cartilage thickness (a), mean (b) and variance (c) of the reflectance score assessed after India ink staining, overall histopathological index of cartilage degeneration (d) and surface irregularity assessed by histopathological grading (e) from age-matched donors of cartilage with articular surfaces that were macroscopically normal (NLA), mildly fibrillated (DGN), and mildly fibrillated from joints undergoing total knee replacement (OA). *p<0.05, **p<0.01, ***p<0.005 versus NLA samples.
Biomechanical Properties
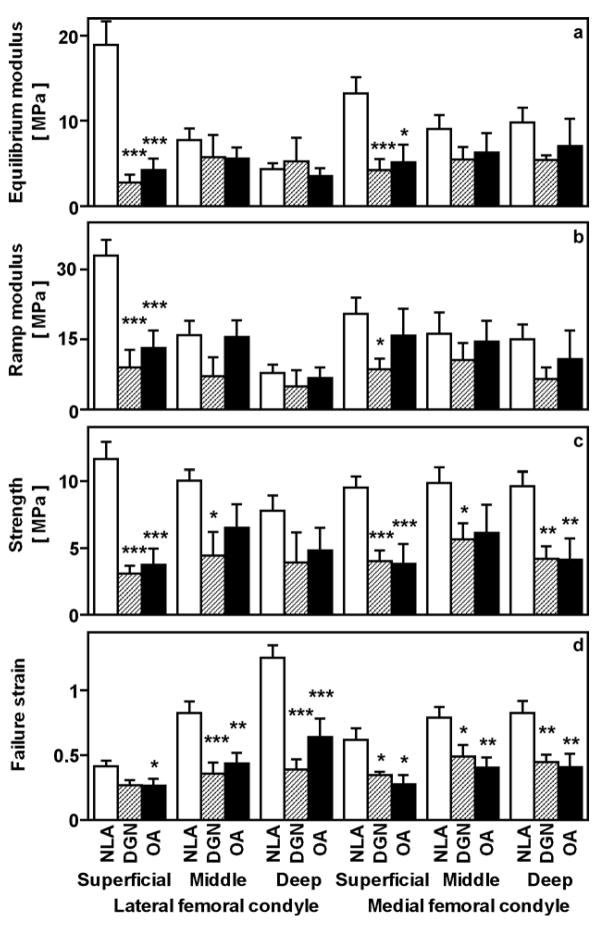
The tensile biomechanical properties of NLA, DGN, and OA cartilage samples varied dramatically. Overall, ramp modulus (Figure 2b), strength (Figure 2c), and failure strain (Figure 2d) were depth-dependent (p<0.005, p<0.05, and p<0.005, respectively), and equilibrium modulus (Figure 2a) tended to be depth-dependent (p=0.2). Tensile ramp modulus, strength, and failure strain tended to be lower in the MFC than the LFC (p=0.06, p=0.06, and p=0.09, respectively), while equilibrium modulus was similar between sites (p=0.6). Specific group differences, as a function of site and depth, are summarized below.
Figure 2.
Tensile biomechanical properties of samples described in Figure 1. For specimens from the superficial, middle, and deep layers, the tensile equilibrium modulus (a), tensile ramp modulus (b), tensile strength (c), and failure strain (d) were determined from equilibrium and then non-equilibrium failure testing of articular cartilage from aged NLA, DGN, and OA donors. *p<0.05, **p<0.01, ***p<0.005 versus NLA samples.
At the LFC and MFC sites, tensile properties varied distinctly between NLA, DGN, and OA experimental groups at different tissue depths. In the superficial layer, the tensile strength was higher in the NLA group than either the DGN or OA groups. In particular, tensile strength of the superficial layer was higher in NLA than DGN and OA samples at both the LFC (each p<0.005) and MFC sites (p<0.005 and p<0.05, respectively). In the middle layer, strength was higher in NLA than DGN samples at both sites (each p<0.05) and tended to be higher than OA samples at the LFC (p=0.08) and MFC (p=0.1) sites. In the deep layer, strength was similar for experimental groups in the LFC (p=0.1–0.7), but higher in NLA than DGN samples (p<0.01) and OA samples (p<0.005) in the MFC. Differences in tensile equilibrium and ramp moduli between experimental groups followed trends similar to that of tensile strength (supplement). Strain (distensibility) at failure for the MFC and the LFC, in particular, was higher in NLA than either DGN or OA samples in the superficial, middle, and deep layers (each p ≤ 0.05). Thus, compared to the NLA group, DGN and OA groups exhibited tensile softening and weakening in a depth-varying pattern, with strength decreased markedly in the superficial zone, and strain increased markedly in the deep zone.
Biochemical Properties
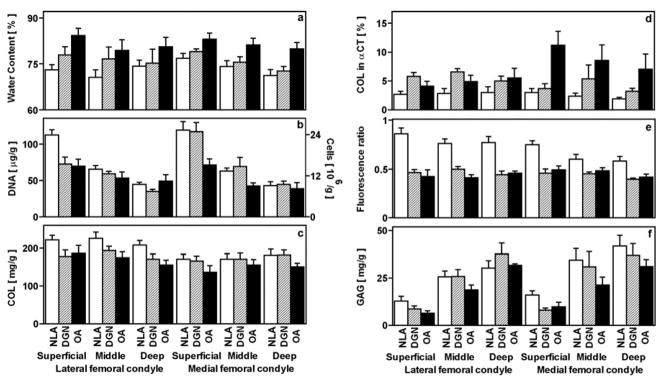
Variations in biochemical constituents with experimental group were dependent strongly on depth and, for some components, on site. Water (p<0.05, Figure 3a), DNA (p<0.005, Figure 3b), and GAG (p<0.005, Figure 3f) contents were dependent on depth, while COL content (p=0.9, Figure 3c), and the fluorescence ratio (p=0.09, Figure 3e) were not. COL in αCT (p=0.08, Figure 3d) tended to be dependent on depth. In particular, water and DNA contents were higher and GAG was lower by 4%, 129%, and 69%, respectively, in the superficial than the deep layer. Water, DNA, COL, and GAG contents were not dependent on site (p=0.1–0.6). The fluorescence ratio was 10% higher in the LFC than the MFC (p<0.01), and COL in αCT tended to be lower in the LFC than the MFC (p=0.07). Distinct site and depth-dependent variations between experimental groups are detailed below.
Figure 3.
Biochemical properties of human articular cartilage samples described in Figure 1. Cartilage tissue adjacent to the mechanical test specimens was analyzed for water content (a), DNA and calculated cell number (b), COL (c), COL in αCT (d), the fluorescence ratio of pentosidine-associated fluorescence (Ex 355/Em 385 nm) to pyridinoline-associated fluorescence (Ex 295/Em 395 nm) (e), and GAG (f). DNA, COL, and GAG were each normalized to wet weight. *p<0.05, **p<0.01, ***p<0.005 versus NLA samples. †p<0.05, ††p<0.01 versus DGN samples.
Water content was higher in OA cartilage than NLA cartilage in most layers of both the LFC and MFC. In the LFC, water content was higher in OA (p<0.005) but not DGN samples (p=0.1) than NLA samples in the superficial layer, and neither DGN nor OA samples were higher than NLA samples in the middle (p=0.2, p=0.09, respectively) or deep layers (p=0.1, p=0.2, respectively). However, in the MFC, water content was higher or tended to be higher in OA and DGN samples than NLA samples, in the superficial (p<0.005, p=0.1, respectively), middle (p<0.025, p=0.08, respectively), and deep layers (p<0.01, p<0.05, respectively).
Variations in DNA content were most dramatic in the superficial layer. In the LFC, DNA content was lower in the superficial layer of DGN and OA samples (each p<0.005) compared to that of NLA samples; DNA content was lower in OA samples than NLA samples (p<0.05) but not DGN samples (p=0.2) in the middle layer, and not different between OA, DGN, or NLA samples in the deep layer (p=0.3–0.8). In the MFC, DNA content in the superficial and middle layers was lower in OA samples than in NLA samples (p<0.005, p<0.05, respectively) and tended to be lower in OA than DGN samples (p<0.05, p=0.07, respectively); in the deep layer, there was little variation between experimental groups (p=0.6–0.8). Thus, DNA content was decreased markedly with cartilage degeneration, especially in the superficial layer.
Differences in the collagen network were evident in COL content, COL in αCT, and the fluorescence ratio. In the LFC, there was a tendency for COL content of NLA samples to be higher than DGN and OA samples in superficial (p=0.08, p=0.1, respectively), middle (p=0.2, p<0.05, respectively), and deep (p=0.08, p<0.01, respectively) layers. In the MFC, COL content varied little in superficial, middle, and deep layers (p=0.1–1.0).
In the LFC, COL in αCT was higher in DGN than NLA samples in superficial (p<0.005) and middle (p<0.01) but not deep (p=0.1) layers, while that in OA samples was similar to that in NLA samples in all layers (p=0.2–0.3). In the MFC, the OA samples displayed a notable amount of COL in αCT, being higher than NLA samples in superficial (p<0.01), middle (p<0.05), and deep (p<0.05) layers and being higher in the superficial layer (p<0.025) but tending not to be higher in the middle or deep layers (p=0.08–0.3) than DGN samples.
There was a striking degeneration-associated decrease in the fluorescence ratio, especially in the LFC. The fluorescence ratio in the LFC was lower in DGN and OA samples than in NLA samples in superficial, middle, and deep layers (each, p<0.005). In the MFC, the fluorescence ratio was or tended to be lower in DGN and OA samples than in NLA samples in the superficial (each, p<0.005), middle (p=0.05–0.1) and deep (each, p<0.025) layers. Taken together, the changes in the structure of the collagen network and the fluorescence ratio indicate remodeling and coincide with the decrease in tensile integrity of cartilage in early degeneration.
GAG content was slightly lower with the onset of early cartilage degeneration. In the LFC, GAG content did not vary between NLA, DGN, and OA samples in the superficial, middle, and deep layers (p=0.08–1.0). In the MFC, GAG content was higher in the superficial layer of NLA samples than DGN (p<0.025) and OA samples (p<0.05) but varied little in the middle and deep layers (p=0.1–0.6).
Relationships Between Mechanical, Structural, and Biochemical Properties
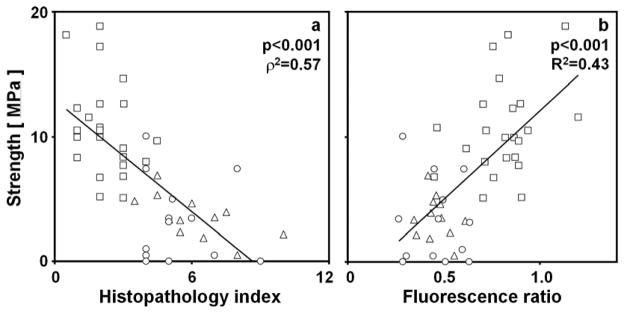
Tensile strength of cartilage layers correlated with certain structural and biochemical properties. Tensile strength of the superficial layer decreased with increasing histopathological index (ρ2=0.57, p<0.001, Figure 4a) and decreased with the fluorescence ratio of the superficial layer (R2=0.43, p<0.001, Figure 4b). The results of additional univariate and multivariate regression are described in the supplement.
Figure 4.
Relationships between tensile strength and structural and biochemical properties of the superficial layer of human articular cartilage of the LFC and MFC. The relationship between tensile strength of the superficial layer and (a) the histopathological index was assessed using Spearman’s rank method to determine p and ρ2 values. The relationship between tensile strength and (b) the fluorescence ratio of the superficial layer was assessed using univariate linear regression to determine p and R2 values. Data are shown for NLA (□), DGN (△), and OA (○) samples. Lines represent the linear regression fits of the data and are shown only to indicate trends.
DISCUSSION
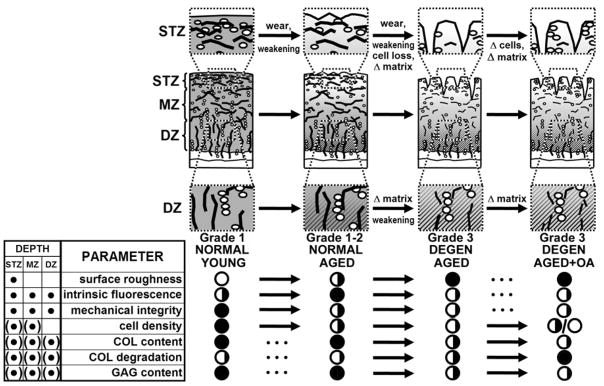
This study of age-matched human articular cartilage delineated the variation in tensile integrity occurring with cartilage surface roughening in non-OA joints and with overt cartilage fibrillation in non-OA joints and OA joints, along with variations in structural and biochemical factors that have been hypothesized to cause cartilage deterioration. The results suggest distinct stages of cartilage degeneration, early–NLA, advanced–DGN, and late–OA, that are prominent at certain anatomical locations and depths (summarized in Figure 5). These stages coincided with variations in the hypothesized causes of biomechanical weakening of articular cartilage, including the degradation and loss of matrix components, collagen network remodeling, decreased cellularity, and mechanical wear.
Figure 5.
Summary of properties of human articular cartilage related to biomechanical deterioration with aging2,3 and osteoarthritis. Histological depiction of mechanical integrity (degree of gray shading), articular surface fibrillation, chondrocyte density, collagen network alteration (fragmentation of fibrils and decrease in intrinsic fluorescence), and loss of GAG (▨) are shown. Tabulated are location of changes, superficial tangential zone (STZ), middle zone (MZ) and deep zone (DZ), denoted by • or (•) for variable changes. Absence (○) or full presence (●) of parameters are indicated under stages noted, with changes indicated by ➞.
Early to advanced-stage transition involved a loss of biomechanical integrity, an increase in surface fibrillation, and a loss of intrinsic fluorescence. Tensile integrity (described by the tensile strength and failure strain) were markedly lower in DGN and OA than NLA samples, differences paralleled by higher indices of surface fibrillation as well as collagen degradation (COL in αCT) and network remodeling (fluorescence ratio) in DGN and OA than NLA samples. These differences may underlie the tensile weakening exhibited by DGN and OA samples (Figure 4a,b).
Advanced to late-stage transition involved an increase in cartilage water content and loss of collagen. Water content was higher (Figure 3a), and COL content (Figure 3c) was lower in OA samples at a stage subsequent to that when tensile integrity was diminished, surface structure altered, collagen degraded, and the network remodeled. The increase in water content and loss of collagen, characteristic features of OA, do not appear to instigate biomechanical weakening since the latter was already present with advanced cartilage deterioration.
The present analysis of tensile properties clarified results from previous studies by analyzing age- and site-matched samples and assessing the role of hypothesized mediators of cartilage deterioration. Age has confounding effects on the study of cartilage degeneration because of the increased prevalence of OA with age1. The tensile moduli, strength, and failure strain, determined in the present study from a substantial sample set of aged adult knees (n=34), together with our previous study of adult knees from different age groups3 allows further interpretation of a previous study of tensile properties4; there, the ~80% lower tensile equilibrium modulus of surface zone cartilage from old-age OA joints compared to young normal joints can be estimated to result from an 18% decrease associated with age (from average ages of 33 to 69 yrs3) combined with a 62% decrease associated with cartilage degeneration (NLA to DGN, Figure 2a).
Site-specific differences were striking and consistent with stages of cartilage degeneration occurring earlier in the MFC than the LFC. These site-specific differences and their variation with age were studied in detail previously in normal cartilage3. While surface roughness was mild in NLA samples, it was greater in the MFC than LFC. This was accompanied by NLA samples of the MFC being generally weaker than those of the LFC. The lower fluorescence in NLA samples of the MFC middle and deep layers compared to LFC samples, but similarity in fluorescence in younger adult samples from the MFC and LFC3 suggests aging-associated differences between these sites. These differences may reflect responses to compressive and shear stress which are higher in the medial than lateral compartment of the knee27 and differences in meniscus contact28. Taken together, these results are consistent with gross pathological analysis29,30, demonstrating a higher prevalence of degeneration in the MFC and earlier and more severe degeneration in the MFC than LFC.
Decreased cellularity observed in this study supports the idea that a decrease in cell density in overtly fibrillated cartilage in DGN and OA samples is associated with cartilage weakening in a site-dependent manner. In the LFC, cellularity (Figure 3b) decreased in the superficial layer at a stage coincident with tensile weakening (Figure 2). In the MFC, however, cellularity was lower at a stage subsequent to a marked decrease in tensile integrity. The decreased DNA is consistent with histological analysis of fibrillated condylar cartilage compared to age-matched macroscopically normal cartilage, especially in the superficial layer14. While age-matched samples were chosen, the variation in cellularity may be due to a combination of cartilage deterioration (NLA to DGN to OA) as well as to aging since cellularity even in macroscopically normal cartilage decreases ~10 31–36% 14 between the ages of 60 and 90yrs. The decrease in cartilage cells and cell density with degeneration may be due to both tissue loss and cell death. The biochemical analysis of DNA indicated that most was within cells rather than in the extracellular space, as the DNA in guanidine and alpha-chymotrypsin extracts (which remove ~90% of GAG matrix) was small (<2% in NLA samples and <8% in DGN and OA samples, data not shown). In fibrillated cartilage, there may be regions of both cell cloning and other regions of hypo-cellularity associated with cell death14,32. The altered metabolic activity of cells in clusters may have a variety of effects on matrix composition and function33.
Several parameters were indicative of active matrix remodeling and loss of collagen matrix, occurring with advanced cartilage degeneration and OA. The higher amount of degraded collagen in DGN and OA samples than NLA samples may result from increased enzymatic activity causing cleavage and denaturation of the collagen network34. The values obtained here (Figure 3d) agree with previous studies23,34 and expand upon those by delineating site and depth-specific variations in age-matched NL, DGN, and OA tissue. The higher COL in αCT in OA samples of the MFC, as well as the lower fluorescence ratio of DGN and OA samples compared to NLA throughout the depth of cartilage, suggest active chondrocyte remodeling involving increased collagen synthesis35 and degradation12. Activated chondrocytes may undergo phenotypic changes resulting in the synthesis of collagen other than type II,36 possibly contributing to the hydroxyproline index of collagen measured here. The similarity in many of the measured properties of DGN and OA samples suggests they are insensitive to the overall condition of the joint, unlike OA-associated changes in chondrocyte metabolism37,38 and ageing-associated changes in chondrocyte metabolism as well as certain extacellular matrix properties38 not assessed here. Such analyses may be useful to further characterize and distinguish NLA, DGN, and OA tissues. The site-specific variations in COL in αCT of OA samples are consistent with more severe degeneration in the MFC. The fluorescence ratio appears to be a simple and sensitive marker of collagen network remodeling, which may result in a functionally inferior collagen network.
The causes of the tensile biomechanical dysfunction of articular cartilage with progressive degeneration and osteoarthritis are consistent with contributions of mechanical and chemical processes such as wear and aberrant metabolism, individually and in combination. Wear-related alteration of tissue structure may be a major contributor (Figure 5); tensile strength in the superficial layer and histopathological index exhibit a strongly inverse relationship (Figure 4a). Collagen network remodeling may also be a major contributor (Figure 5), with a strong positive relationship between tensile strength and fluorescence ratio (Figure 4b). Compared to NLA samples, DGN and OA samples in superficial, middle, and deep layers exhibit concomitant tensile weakening (Figure 2), collagen degradation (Figure 3d), and lowering of intrinsic fluorescence (Figure 3e). The increase in water content, but lack of a change in cartilage thickness, may be indicative of wear combined with cartilage tissue swelling, or, alternatively, the loss of matrix components throughout the full thickness of the tissue associated with cartilage fibrillation. Additional studies are needed to elucidate the mechanisms behind surface fibrillation and collagen network remodeling and their role in biomechanical dysfunction and the development and progression of OA.
Acknowledgments
Support: Supported by research grants from the National Institutes of Health, the National Science Foundation, and a grant to UCSD, in support of RLS, from the Howard Hughes Medical Institute through the HHMI Professors Program, and also by Sharp Healthcare, San Diego, CA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Praemer A, Furner S, Rice DP. Musculoskeletal Conditions in the United States. Park Ridge, IL: American Academy of Orthopaedic Surgeons; 1999. [Google Scholar]
- 2.Kempson GE. Relationship between the tensile properties of articular cartilage from the human knee and age. Ann Rheum Dis. 1982;41:508–511. doi: 10.1136/ard.41.5.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Temple MM, Bae WC, Chen MQ, Lotz M, Amiel D, Coutts RD, et al. Age- and site-associated biomechanical weakening of human articular cartilage of the femoral condyle. Osteoarthritis Cartilage. 2007;15:1042–1052. doi: 10.1016/j.joca.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Akizuki S, Mow VC, Muller F, Pita JC, Howell DS, Manicourt DH. Tensile properties of human knee joint cartilage: I. influence of ionic conditions, weight bearing, and fibrillation on the tensile modulus. J Orthop Res. 1986;4:379–392. doi: 10.1002/jor.1100040401. [DOI] [PubMed] [Google Scholar]
- 5.Kempson GE, Muir H, Pollard C, Tuke M. The tensile properties of the cartilage of human femoral condyles related to the content of collagen and glycosaminoglycans. Biochim Biophys Acta. 1973;297:456–472. doi: 10.1016/0304-4165(73)90093-7. [DOI] [PubMed] [Google Scholar]
- 6.Bae WC, Wong VW, Hwang J, Antonacci JM, Nugent-Derfus GE, Blewis ME, et al. Wear-lines and split-lines of human patellar cartilage: relation to tensile biomechanical properties. Osteoarthritis Cartilage. 2008;16:841–845. doi: 10.1016/j.joca.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Squires GR, Okouneff S, Ionescu M, Poole AR. The pathobiology of focal lesion development in aging human articular cartilage and molecular matrix changes characteristic of osteoarthritis. Arthritis Rheum. 2003;48:1261–1270. doi: 10.1002/art.10976. [DOI] [PubMed] [Google Scholar]
- 8.Bader DL, Kempson GE, Barrett AJ, Webb W. The effects of leucocyte elastase on the mechanical properties of adult human articular cartilage in tension. Biochim Biophys Acta. 1981;677:103–108. doi: 10.1016/0304-4165(81)90150-1. [DOI] [PubMed] [Google Scholar]
- 9.Bader DL, Kempson GE, Egan J, Gilbey W. The effects of selective matrix degradation on the short-term compressive properties of adult human articular cartilage. Biochim Biophys Acta. 1992;116:147–154. doi: 10.1016/0304-4165(92)90111-7. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt MB, Mow VC, Chun LE, Eyre DR. Effects of proteoglycan extraction on the tensile behavior of articular cartilage. J Orthop Res. 1990;8:353–363. doi: 10.1002/jor.1100080307. [DOI] [PubMed] [Google Scholar]
- 11.Nelson F, Dahlberg L, Laverty S, Reiner A, Pidoux I, Ionescu M, et al. Evidence for altered synthesis of Type II collagen in patients with osteoarthritis. J Clin Invest. 1998;102:2115–2125. doi: 10.1172/JCI4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson GJ, Verner JJ, Nelson FR, Lin DL. Degradation of the cartilage collagen matrix associated with changes in chondrocytes in osteoarthrosis. Assessment by loss of background fluroescence and immunodetection of matrix components. J Orthop Res. 2001;19:33–42. doi: 10.1016/S0736-0266(00)00008-5. [DOI] [PubMed] [Google Scholar]
- 13.Eyre DR, McDevitt CA, Bilingham MEJ, Muir H. Biosynthesis of collagen and other matrix proteins by articular cartilage in experimental osteoarthrosis. Biochem J. 1980;188:823–837. doi: 10.1042/bj1880823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitrovic D, Quintero M, Stankovic A, Ryckewaert A. Cell density of adult human femoral condylar articular cartilage. Lab Invest. 1983;49:309–316. [PubMed] [Google Scholar]
- 15.Meachim G. Light microscopy of Indian ink preparations of fibrillated cartilage. Ann Rheum Dis. 1972;31:457–464. doi: 10.1136/ard.31.6.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada K, Healey R, Amiel D, Lotz M, Coutts R. Subchondral bone of the human knee joint in aging and osteoarthritis. Osteoarthritis Cartilage. 2002;10:360–369. doi: 10.1053/joca.2002.0525. [DOI] [PubMed] [Google Scholar]
- 17.Bae WC, Temple MM, Amiel D, Coutts RD, Niederauer GG, Sah RL. Indentation testing of human cartilage: sensitivity to articular surface degeneration. Arthritis Rheum. 2003;48:3382–3394. doi: 10.1002/art.11347. [DOI] [PubMed] [Google Scholar]
- 18.Kempson GE, Spivey CJ, Swanson SA, Freeman MA. Patterns of cartilage stiffness on normal and degenerate human femoral heads. J Biomech. 1971;4:597–609. doi: 10.1016/0021-9290(71)90049-2. [DOI] [PubMed] [Google Scholar]
- 19.Williamson AK, Chen AC, Masuda K, Thonar EJ-MA, Sah RL. Tensile mechanical properties of bovine articular cartilage: variations with growth and relationships to collagen network components. J Orthop Res. 2003;21:872–880. doi: 10.1016/S0736-0266(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 20.McGowan KB, Kurtis MS, Lottman LM, Watson D, Sah RL. Biochemical quantification of DNA in human articular and septal cartilage using PicoGreen and Hoechst 33258. Osteoarthritis Cartilage. 2002;10:580–587. doi: 10.1053/joca.2002.0794. [DOI] [PubMed] [Google Scholar]
- 21.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 22.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 23.Bank RA, Krikken M, Beekman B, Stoop R, Maroudas A, Lafeber FPJG, et al. A simplified measurement of degraded collagen in tissues: application in healthy, fibrillated and osteoarthritic cartilage. Matrix Biol. 1997;16:233–243. doi: 10.1016/s0945-053x(97)90012-3. [DOI] [PubMed] [Google Scholar]
- 24.Stockwell RA. The cell density of human articular and costal cartilage. J Anat. 1967;101:753–763. [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson DS, Cleary EG. The determination of collagen and elastin. Methods Biochem Anal. 1967;15:25–76. doi: 10.1002/9780470110331.ch2. [DOI] [PubMed] [Google Scholar]
- 26.Sokal RR, Rohlf FJ. Biometry. 3. New York: WH Freeman and Co; 1995. [Google Scholar]
- 27.Morrison JB. The mechanics of the knee joint in relation to normal walking. J Biomech. 1970;3:51–61. doi: 10.1016/0021-9290(70)90050-3. [DOI] [PubMed] [Google Scholar]
- 28.Freeman MA, Pinskerova V. The movement of the normal tibio-femoral joint. J Biomech. 2005;38:197–208. doi: 10.1016/j.jbiomech.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Bennett GA, Waine H, Bauer W. Changes in the Knee Joint at Various Ages with Particular Reference to the Nature and Development of Degenerative Joint Disease. New York: The Commonwealth Fund; 1942. [Google Scholar]
- 30.Muehleman C, Bareither D, Huch K, Cole AA, Kuettner KE. Prevalence of degenerative morphological changes in the joints of the lower extremity. Osteoarthritis Cartilage. 1997;5:23–37. doi: 10.1016/s1063-4584(97)80029-5. [DOI] [PubMed] [Google Scholar]
- 31.Quintero M, Mitrovic DR, Stankovic A, de Seze S, Miravet L, Ryckewaert A. Aspects cellulaires du vieillissement du cartilage articulaire. II. cartilage condylien a surface fissuree preleve dans les genoux normaux et arthrosiques. Revue du Rhumatisme. 1984;51:445–449. [PubMed] [Google Scholar]
- 32.Aigner T, Hemmel M, Neureiter D, Gebhard PM, Zeiler G, Kirchner T, et al. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritic human articular knee cartilage. Arthritis Rhuem. 2001;44:1304–1312. doi: 10.1002/1529-0131(200106)44:6<1304::AID-ART222>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 33.Aigner T, Vornehm SI, Zeiler G, Dudhia J, von der Mark K, Bayliss MT. Suppression of cartilage matrix gene expression in upper zone chondrocytes of osteoarthritic cartilage. Arthritis Rheum. 1997;40:562–569. doi: 10.1002/art.1780400323. [DOI] [PubMed] [Google Scholar]
- 34.Hollander AP, Heathfield TF, Webber C, Iwata Y, Bourne R, Rorabeck C, et al. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994;93:1722–1732. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eyre DR, Wu J-J. Collagen structure and cartilage matrix integrity. J Rheumatol. 1995;22S:82–88. [PubMed] [Google Scholar]
- 36.Gibson G, Lin DL, Francki K, Caterson B, Foster B. Type X collagen is colocalized with a proteoglycan epitope to form distinct morphological structures in bovine growth cartilage. Bone. 1996;19:307–315. doi: 10.1016/s8756-3282(96)00222-0. [DOI] [PubMed] [Google Scholar]
- 37.Tsuchiya K, Maloney WJ, Vu T, Hoffman AR, Huie P, Sibley R, et al. Osteoarthritis: differential expression of matrix metalloproteinase-9 mRNA in nonfibrillated and fibrillated cartilage. J Orthop Res. 1997;15:94–100. doi: 10.1002/jor.1100150114. [DOI] [PubMed] [Google Scholar]
- 38.Aigner T, Haag J, Martin J, Buckwalter J. Osteoarthritis: aging of matrix and cells--going for a remedy. Curr Drug Targets. 2007;8:325–331. doi: 10.2174/138945007779940070. [DOI] [PubMed] [Google Scholar]