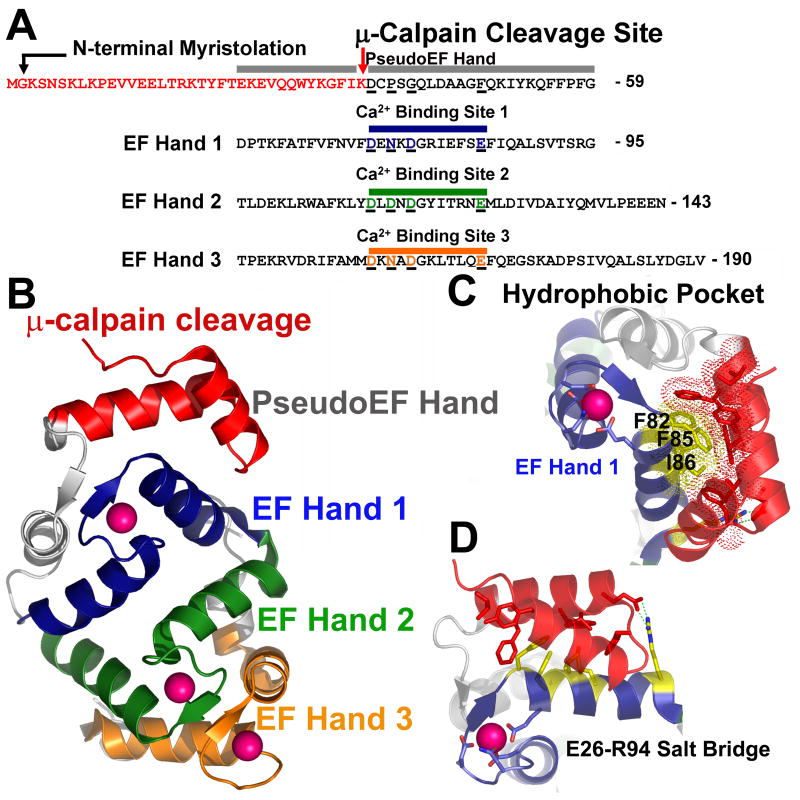
Fig. 4. μ-calpain cleavage of NCS-1 directly impacts Ca2+ binding through destabilization of EF-hand 1, exposure of hydrophobic surface, and possible disruption of the overall NCS-1 fold.
A. Schematic of the sequence of NCS-1. Colored red are residues removed by μ-calpain cleavage. Residues which encompass the pseudoEF hand, EF hand 1, EF hand 2, and EF hand 3, along with corresponding Ca2+ binding sites are aligned and color coded. The non-functional pseudoEF hand is colored grey. The Ca2+ binding loop of EF hand 1 is colored blue, EF hand 2 green and EF hand 3 orange. Acidic residues coordinating Ca2+ in each of these EF hand domains are underlined and colored accordingly. Corresponding residues in the pseudo-EF hand which do not bind Ca2+ are also aligned and underlined for comparison. B. Overall structure of NCS-1. Residues removed by μ-calpain within the pseudoEF hand are colored red. The pseudoEF hand is labeled grey. EF hand 1 is colored blue, EF hand 2 is colored green, and EF hand 3 is colored orange. Bound Ca2+ ions are depicted as pink spheres. C. Hydrophobic pocket centered on F82, F85 and I86 in NCS-1 (colored yellow and shown as sticks with dots indicating van der Waals contacts. Residues forming van der Waals contacts with F82, F85 and I86 which would be removed by μ-calpain cleavage are shown as red sticks. D. Salt bridge formed between R94 of EF hand 1 and E26 of the pseudoEF-hand would be disrupted by μ-calpain cleavage possibly destabilizing the conformation of EF hand 1.