Figure 1.
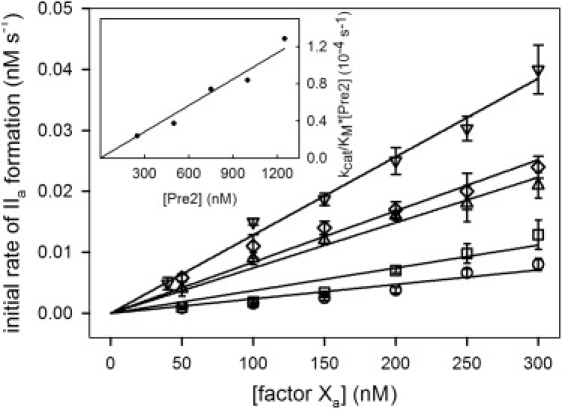
Initial rate of Pre2 activation to thrombin by FXa. The initial rate of thrombin (IIa) formation was determined at 37°C as a function of increasing concentration of FXa at different concentrations of Pre2: [Pre2] = 250 nM (○), 500 nM (□), 750 nM (▵), 1000 nM (♢), and 1250 nM (▿). Initial rates were determined in buffer A (50 mM Tris, 150 mM NaCl, and 0.6% PEG, pH 7.4) containing 4 mM CaCl2, using the substrate S-2238 as described in Experimental Procedures in the Supporting Material. The curves drawn through the symbols are straight lines, the slopes of which are R1 (as described in Appendix A in the Supporting Material) for different [Pre2]. (Inset) Linear dependence of R1 on [Pre2], which demonstrates that the reaction follows Michaelis-Menten kinetics under our conditions and gives, as the slope of this plot, kcat/KM = 94 ± 2 M−1 s−1 for free FXa. By globally fitting all five data sets to the Michaelis-Menten model (20), we obtained the same kcat/KM as well as KM = (10 ± 1) mM.
