Figure 3.
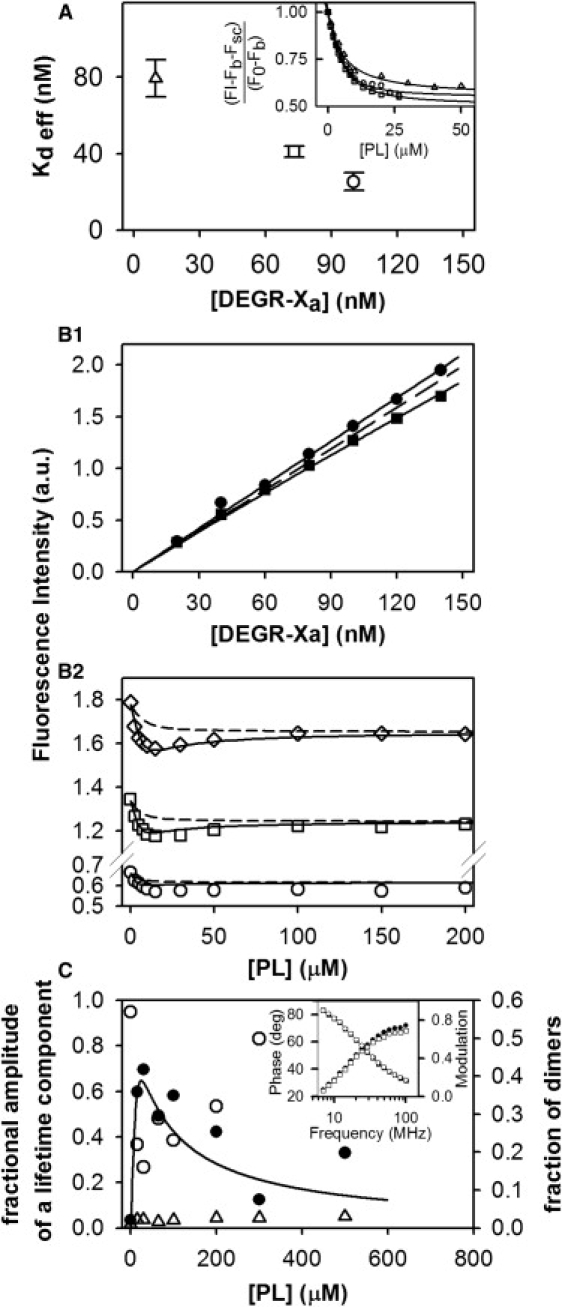
Fluorescence intensity studies of DEGR-Xa. (A) DEGR-Xa binding to membranes depends on DEGR-Xa concentration. (Inset) DEGR-Xa binding to 25% PS/DOPC membranes ([PL]) was monitored at 23°C as a relative fluorescence change as PL was added at different concentrations of DEGR-Xa (▵, 10 nM; □, 73 nM; and ○, 100 nM) in buffer A containing 2 mM Ca2+. (Main plot) Open symbols show apparent dissociation constants for DEGR-Xa binding obtained from the data shown in the inset fit (inset symbols are consistent with main plot) to a simple binding model (8). (B1 and B2) Global analysis of DEGR-Xa membrane binding using either (B1) DEGR-Xa or (B2) membranes as titrant. (B1) Fluorescence intensity of DEGR-Xa (scattering and background subtracted) in Buffer B containing 4 mM CaCl2 at 23°C as a function of increasing DEGR-Xa concentration at different concentrations (●, 0 μM; and ■, 15 μM) of 25% PS/DOPC membranes. (B2) Fluorescence intensity of DEGR-Xa (○, 50 nM; □, 100 nM; and ♢, 150 nM) at increasing concentrations of 25% PS/DOPC membranes. Data in both frames were fitted globally to the dimerization model (Appendices A and B in the Supporting Material) (solid lines), yielding a surface dimer dissociation constant × 10−9 mol/(dm)2 for DEGR-Xa, which corresponds to a solution dimer dissociation constant μM calculated at [PL] = 10 μM. Dotted lines show fits to a model that assumes that DEGR-Xa can bind to membranes only in its monomer form. Reduced χ-square for the global fit equals , and normalized χ-square , The p value for the null hypothesis is <0.02. (C) DEGR-Xa fluorescence lifetime during titration with 25:75 PS/DOPC membranes. DEGR-Xa (300 nM) was titrated with 25% PS/DOPC membranes in buffer B containing 4 mM Ca2+ at 23°C, and its fluorescence lifetime was resolved into three lifetime components using software supplied with the Spex FluoroLog-3 phase and modulation spectrofluorometer (HORIBA Jobin Yvon, Edison, NJ). Fractional amplitudes of lifetime components with lifetime τ ≈ 7.4 ns (●), 13 ns (○), and 0.5 ns (▵) are plotted, along with the relative proportion of DEGR-Xa in dimer form (solid line) as predicted by the dimer model using the dimerization constant obtained from the global fit shown in panel B. (Inset) Phase and modulation of the signal measured at low lipid concentration [PL] = 15 μM (●, phase; ■, modulation) and high lipid concentration [PL] = 300 μM (○, modulation; □, phase).
