Figure 4.
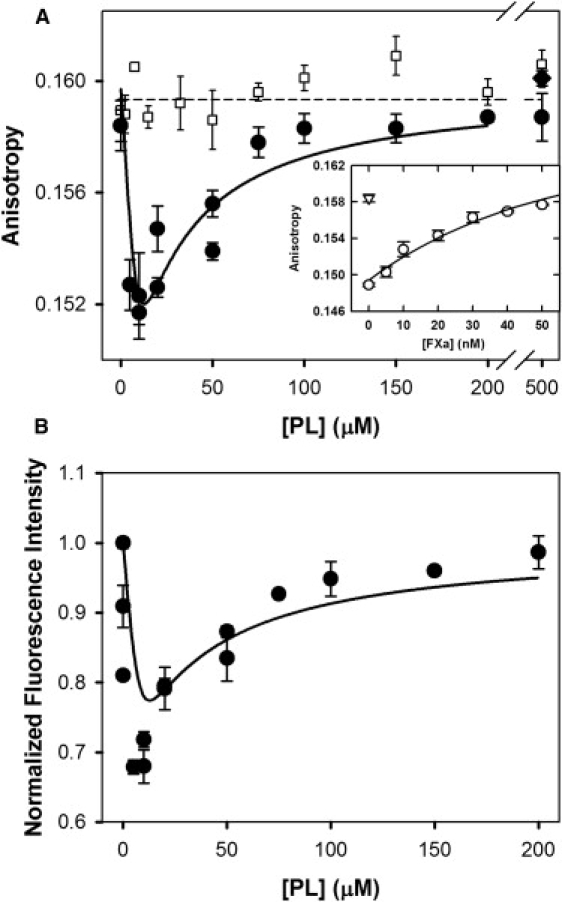
FEGR-Xa fluorescence during titration with 25:75 PS/DOPC membranes. (A) FEGR-Xa (150 nM) in buffer B containing 1 mM Ca2+ (□) or 4 mM Ca2+ (●) was titrated with 25% PS/DOPC membranes, and its fluorescence anisotropy is plotted versus PS-containing membrane concentration ([PL]) before (□ and ●) and after (♦) addition of detergent. (Inset) Fluorescence anisotropy increase of FEGR-Xa after addition of increasing amounts of FXa in the presence of 10 μM 25% PS/DOPC membrane. Anisotropy was fitted using a model similar to the one in Appendix B in the Supporting Material with the addition of competition between FXa and FEGR-Xa. The data were fitted with only one parameter: for FEGRXa-FXa dimer, which was similar to the dimer dissociation constant for FXa dimer; all other parameters were used as obtained from previously described experiments. Obtained reduced χ-square is 2.98, and normalized χ-square is 1.73. The p value for the null hypothesis is <0.01. (B) Fluorescence intensity (relative to intensity at [PL] = 0) of FEGR-Xa is plotted versus PS-containing membrane concentration ([PL]) in buffer containing 4 mM Ca2+ (●). Solid lines through the data in both frames were obtained from the best global fit of the dimer model to the data, as described in Appendix C in the Supporting Material. The dashed line in panel A shows the fraction of FEGR-Xa present as monomer plus free species (i.e., 1-Xdimer), as derived using × 10−9 mol/(dm)2 as obtained from the global fit. Reduced χ-square for the global fit equals , and normalized χ-square . The p value for the null hypothesis is <0.02.
