1. Structure
RDS, the protein product of the retinal degeneration slow (RDS) gene (also called PRPH2 ENSG00000112619) was first identified by Robert Molday’s group in 1987. The gene was cloned two years later by Gabriel Travis’ group and was shown to be the gene responsible for the phenotype in the retinal degeneration slow (rds) mutant mouse which was first described in 1978. RDS is a 346aa, 37kDa tetraspanin glycoprotein expressed exclusively in the rod and cone photoreceptor outer segments (OSs). Within the OS, RDS expression is limited to the hairpin-like rim region of rod discs/incisures and cone lamellae (Goldberg, 2006).
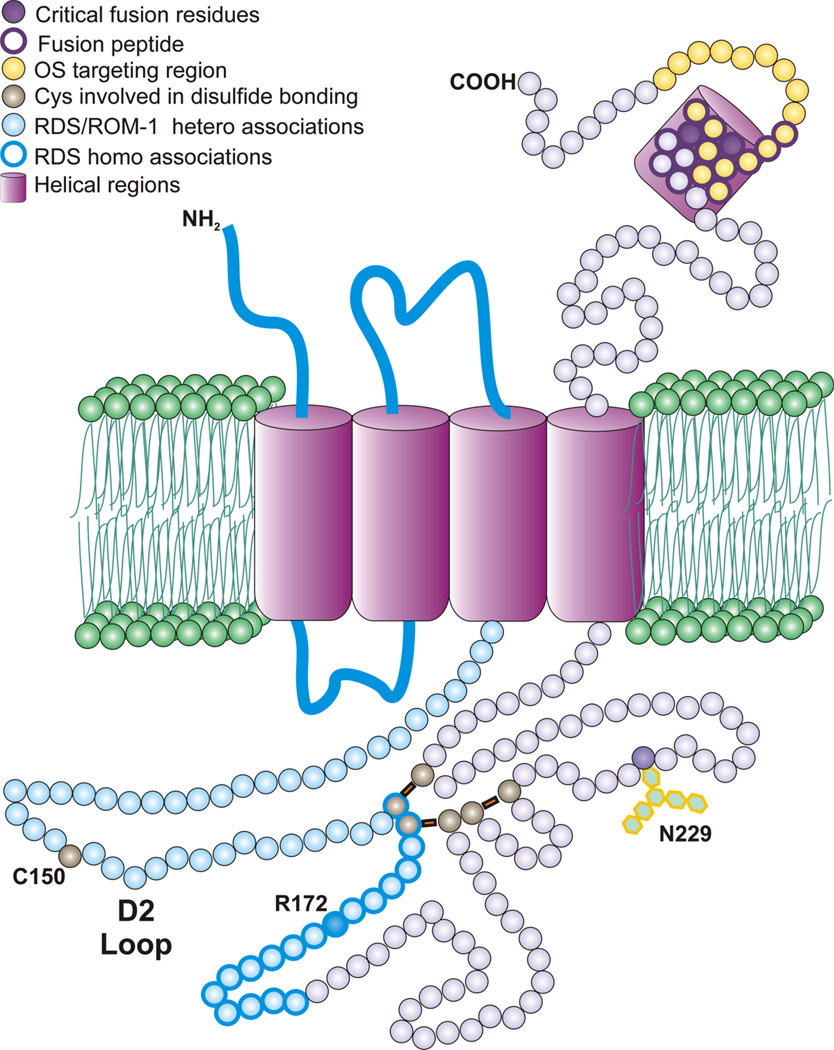
RDS is an integral membrane protein with four transmembrane domains, cytosolic N-and C-termini, and a large extracellular/intradiscal D2 loop (Fig. 1). The majority of the C-terminus has been shown to be intrinsically disordered, although small portions take the form of amphipathic helices. Although the D2 loop region contains six cysteines involved in intermolecular disulfide bonding, in common with other tetraspanins, it also contains a unique, seventh unpaired cysteine which is involved in RDS complex assembly. RDS has a homologue, rod outer segment membrane protein 1 (ROM-1) which shares many of its structural features. In contrast to ROM-1, RDS is glycosylated at N229, but studies have shown that this glycosylation is not required for normal protein function.
Figure 1.
Schematic representation of the RDS polypeptide with various important features annotated.
In the photoreceptor inner segment, RDS and ROM-1 assemble non-covalently into homo- and hetero-tetramers. These core complexes then traffic to the OS where they are further assembled into octamers and higher-order oligomers. While tetramers and octamers contain both RDS and ROM-1, higher-order oligomers contain only RDS. These higher order oligomers are held together by disulfide bonds mediated by the seventh cysteine (150) of the D2 loop of RDS and have been hypothesized to maintain the disc rim structure by bridging disc membranes. The predicted size of two RDS or ROM-1 D2 loops in opposite membranes would be sufficient to bridge the intralumenal distance although no direct evidence exists to support this idea (Goldberg, 2006).
2. Function
The primary function of RDS is the formation of the OS rim region in both rod discs and cone lamellae. Initial studies were conducted using the rds spontaneous knockout mouse and demonstrated that in the rod dominant retina, RDS is required for OS formation; OSs in the rds−/− mice do not form and photoreceptors undergo degeneration. However, in the presence of 50% of the normal complement of RDS, the primary structural defect in the retina is a lack of rim formation. OSs in the rds+/− mice are characterized by large membranous whorls lacking the traditional orderly disc structure and the crucial hairpin structure at the perimeter of the discs. This abnormality is accompanied by deficits in rod function. In both the rds−/− and the rds+/− rod degeneration occurs much earlier and is much more severe than cone degeneration, although cone structure and function also eventually decline.
In addition to this structural role, RDS is hypothesized to assist in the maintenance of OS structure after its formation. RDS has been shown to interact with the GARP portion of the β-subunit of the rod (but not cone) cyclic nucleotide gated ion channel. Since this plasma membrane channel also interacts with phosphodiesterase, it has been suggested that RDS may act as a scaffolding protein of sorts. This function would be in keeping with that of other tetraspanins; many of which have been shown to exert their functions via the organization of a membrane microdomain similar to, but distinct from lipid rafts. The “tetraspanin web” is typically composed of several proteins associated with varying degrees of stringency and can modulate many cellular activities. Two additional RDS functions have been ascribed to partially overlapping regions in the C-terminus of RDS. First, a segment has been identified as that responsible for trafficking of both RDS and ROM-1 (in tetrameric form) to the OS through a rhodopsin independent pathway. Second, recent evidence has indicated that part of the C-terminus functions as a fusion peptide and may work in concert with the membrane fusion protein melanoregulin to seal rod discs at the base of the OS (Boesze-Battaglia, et al., 2007).
Studies from the cone-dominant Nrl−/− retina have suggested that RDS may have a different role in cones than rods. Nrl−/− mice with 50% of the normal amount of RDS (Nrl−/−/rds+/−) have “rimless” OSs characterized by whorls similar to those seen in the rds+/−. However, in striking contrast to the rods of the rds−/−, cones in Nrl−/−/rds−/− mice retain some tubular OS structure (albeit malformed) and are capable of phototransduction (Farjo, et al., 2007). The mechanism underlying this differential function in rods vs. cones is not known. Nrl−/− retinas have RDS and RDS/ROM-1 complexes that are indistinguishable from those in rods so differences in oligomerization are not likely to explain the variation. If RDS is indeed serving a scaffolding function or organizing a membrane microdomain, it is possible that the differences in the composition of such a domain in rods and cones might explain the differences. It is also possible that cones do not have an absolute requirement for RDS for OS formation because the fusogenic role of RDS is not required in the base of the cone OS (which does not form closed fused discs).
3. Disease Involvement
Over 80 different disease causing mutations in RDS have been identified (http://www.retina-international.com/sci-news/rdsmut.htm). Consistent with the experimental observation that RDS functions differently in rods and cones, mutations cause both rod- and cone-dominant disease phenotypes. There is significant variability in disease onset, severity, and phenotype: presentations range from traditional autosomal dominant retinitis pigmentosa to varying macular dystrophies, cone and cone-rod dystrophy, central areolar chroidal dystrophy, retinitis punctata albescens, and autosomal recessive Stargardt disease. Although most RDS-associated retinal disease is thought to be monogenic, a digenic form involving a concurrent ROM-1 mutation has also been reported.
In vivo and in vitro studies on models carrying disease causing mutations have demonstrated that rod-dominant RDS disease usually arises from a loss-of-function mutation and consequent haploinsufficiency phenotype. In contrast, cone dominant disease phenotypes are most often associated with toxic gain-of-function mutations. For example, in a transgenic mouse model carrying the macular dystrophy mutation R172W, cones exhibit a classic gain-of-function phenotype; cone function and structure are significantly perturbed in both the WT and rds+/− backgrounds compared to non-transgenic littermates. Conversely, rods exhibit a hypomorphic phenotype; OS structure and function are improved in R172W/rds+/− rods compared to the haploinsufficient rds+/− rods, but are not fully rescued. These data and those from several other animal models of RDS-associated retinal disease suggest that rod function depends more on the total quantity of RDS present while cone function relies more on the presence of properly structured RDS.
Although treatment for RDS-associated inherited retinal degeneration has been historically limited (and remains so in the clinic), recent advances in both viral and non-viral gene therapy have been promising. Subretinal delivery of RDS cDNA and/or ciliary derived neurotrophic factors to rds+/− and rds−/− mice (packaged in either AAV or compacted DNA nanoparticles) results in partial structural and functional improvement in the haploinsufficiency phenotype (Ali, et al., 2000). Gene therapy based treatments of cone-dominant RDS disease will likely require knockdown therapy coupled with gene replacement, and these approaches are being explored.
4. Future Studies
Future studies on RDS will likely take two directions. The first will be optimizing therapeutic strategies, including designing knockdown therapies for dominant degenerations and improving the rescue achieved with current vectors. The second focus will likely be on understanding how RDS functions differently in rods vs. cones and how those differences may contribute to processes underlying cone vs. rod OS biogenesis. Insight into these differences may come from studies designed to identify the composition of the RDS tetraspanin web, from additional studies of the cellular behavior of cone-dominant and rod-dominant disease causing mutations, and from further investigation of the fusogenic role of RDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Goldberg AF. Role of peripherin/rds in vertebrate photoreceptor architecture and inherited retinal degenerations. Int Rev Cytol. 2006;253:131–175. doi: 10.1016/S0074-7696(06)53004-9. [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Song H, Sokolov M, et al. The tetraspanin protein peripherin-2 forms a complex with melanoregulin, a putative membrane fusion regulator. Biochemistry. 2007;46:1256–1272. doi: 10.1021/bi061466i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farjo R, Fliesler SJ, Naash MI. Effect of Rds abundance on cone outer segment morphogenesis, photoreceptor gene expression, and outer limiting membrane integrity. J Comp Neurol. 2007;504:619–630. doi: 10.1002/cne.21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali RR, Sarra GM, Stephens C. Restoration of photoreceptor ultrastructure and function in retinal degeneration slow mice by gene therapy. Nat Genet. 2000;25:306–310. doi: 10.1038/77068. [DOI] [PubMed] [Google Scholar]