Figure 7.
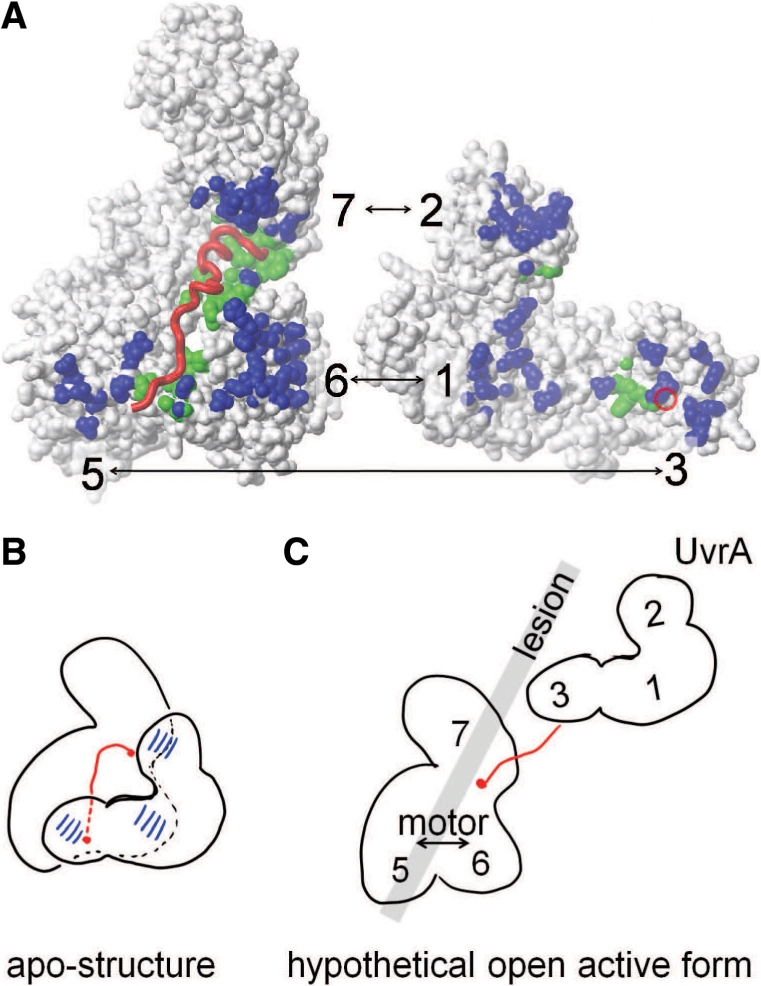
Mechanistic proposal for the role for MfdN:MfdC contacts in regulating Mfd. (A) Interface between MfdN, linker and MfdC in the apo-structure (2EYQ). To afford a view into the interface, coordinates of MfdN were rotated by 180° about a vertical axis and shifted to the right. MfdN and MfdC are shown as white surfaces, and the linker as a red worm. The red circle on the surface of MfdN indicates the N-terminal connection of the linker. Atoms in MfdN closer than 5 Å to atoms in MfdC and vice versa are shown in blue. Atoms in MfdN and MfdC closer than 5 Å to atoms in the linker are shown in green. (B) Schematic of the inter-domain contacts and the path of the linker region through the MfdN:MfdC interface in the apo-conformation. (C) Hypothetical model of an open conformation lacking all MfdN:MfdC interactions. Mfd domains are numbered, and the gray bar indicates the putative path of DNA and the location of the lesion when Mfd binds to a stalled RNAP (the latter is omitted for clarity). In this scenario, the UvrA-binding site in domain 2 is no longer occluded and MfdN is mobile, tethered only by the linker to MfdC. The interactions between the N-terminal portion of the linker with the motor domains 5 and 6 are absent, and the motor domains are no longer restrained by contacts with MfdN. Thus, a switch to the proposed open conformation would activate UvrA recruitment and motor function in a concerted manner.
