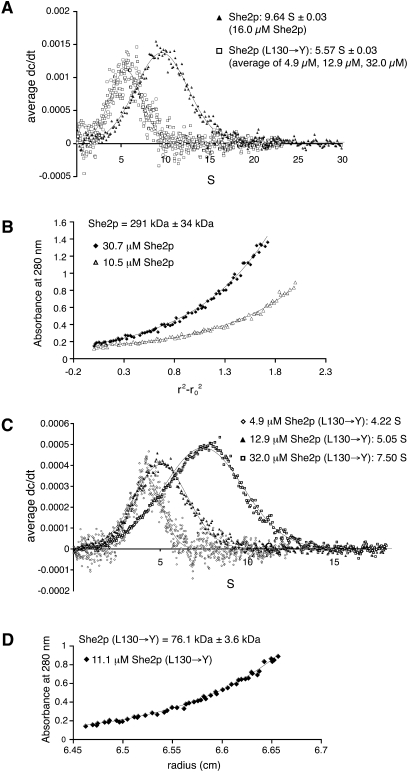
FIGURE 1.
Analytical-ultracentrifugation experiments with She2p. (A) In sedimentation–velocity experiments wild-type She2p sediments at 9.6 S. In contrast, She2p (L130→Y) sedimentation is concentration dependent with an average of 5.6 S (individual experiments are shown in C). The reduced sedimentation coefficient for She2p (L130→Y) is consistent with a reduced molecular weight. (B) Sedimentation–equilibrium experiments with wild-type She2p at different protein concentrations indicate a concentration-independent oligomer of 290 kDa. Plot shows two representative curves. (C) Sedimentation–velocity experiments with She2p (L130→Y) at different protein concentrations show a concentration-dependent change in sedimentation. (D) Sedimentation–equilibrium experiments with She2p (L130→Y) at an intermediate concentration of 11.1 μM revealed a molecular weight of 76 kDa, confirming that higher molecular weight oligomers are disrupted by this mutation.