FIGURE 4.
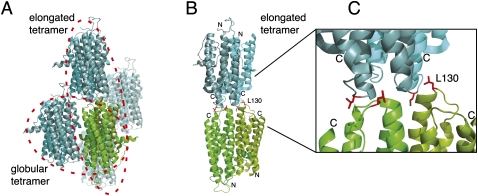
Crystallographic contacts of She2p. (A) In the published crystal structure (PDB ID: 1XLY) a stable She2p dimer (subunits in light and dark green) was observed with a large buried surface interface of over 2000 Å2/monomer (Table 1; Niessing et al. 2004). Among the several crystallographic contacts (subunits shown in light and dark blue, respectively) only two dimer contacts have significant buried surface interfaces (Table 1). In one case, the She2p dimer binds on its flat, basic-helical hairpin-containing side to another dimer in an upside-down orientation. We termed this complex globular tetramer. In the other case, She2p interacts with another dimer through its uncharged upper surface in a head-to-head conformation. This complex was termed elongated tetramer. As defined in Niessing et al. (2004), the upper surface refers to the uncharged area on top of the She2p dimer when oriented with the N-termini to the bottom and the C-termini to the top. (B) The elongated tetramer with leucine 130 shown with its side chains in red and rotated slightly when compared to (A). Leucine 130 is located at the dimer interface of the elongated tetramer. Formation of tetramers is disrupted by mutation of leucine 130 into tyrosine. The positions of N- and C-termini are marked. (C) Close-up of the tetramer–interaction surface shown in (B). Images were generated using PyMOL (DeLano Scientific).