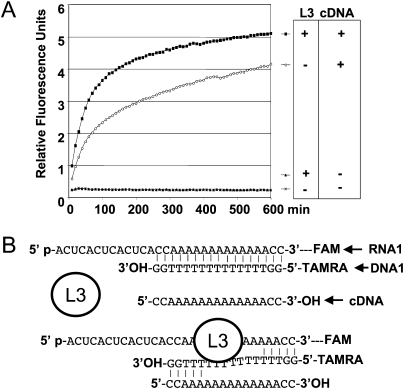
FIGURE 8.
Impact of an RNA binding protein with no nuclease activity. (A) The following assays were monitored on a Rotor Gene apparatus at 30°C, in the conditions described in Figure 1 (identical assay buffer and concentration of 0.5 μM of RNA1 [FAM-linked], 1 μM of DNA1 [TAMRA-linked]). In the presence of the ribosomal protein L3, 100 nM (Redko and Condon 2009), the lack of an increase in FAM fluorescence confirms that the duplex continues to form sufficiently. Thus, the RNA binding of a protein, per se, does not produce a fluorescence signal. However, the addition of 2 μM of a complementary DNA of DNA1, cDNA, used as a trap, leads to an increase in the fluorescence signal, more rapid if L3 is present. Sequence of cDNA is 5′-CCAAAAAAAAAAAAACC-3′. This observation is similar to what is known from the experiments on RNA helicase activity measurements (Bizebard et al. 2004). (B) Schematic representation of how the presence of cDNA and/or L3 can separate the two strands of the RNA1/DNA1 duplex. This approach is potentially adaptable to RNA helicase activity measurements.