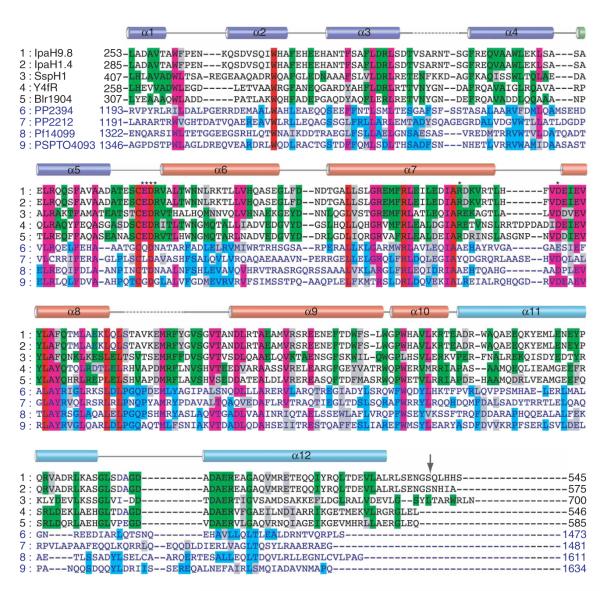
Figure 4.
Multiple sequence alignment of IpaH homologs. The alignment is restricted to the C-terminal domain identical in all IpaH proteins (IpaH-CTD), which spans residues 254 to 540 (indicated by arrow) in IpaH9.8 and residues 285 to 571 in IpaH1.4. A set of nine representative proteins was selected from the alignment of 25 IpaH homologs13 including (1) IpaH9.8 and (2) IpaH1.4 from S. flexneri, (3) SspH1 from Salmonella typhimurium, (4) Y4fR from Rhizobium spp., (5) Blr1904 from Bradyrhizobium japonicum, (6) PP1072 and (7) PP2212 from Pseudomonas putida, (8) Pfl14099 from Pseudomonas fluorescens and (9) PSPTO4093 from P. syringae pv. tomato. Sequences of the C-terminal domain of the ~600-residue proteins (rows 1–5) and ~1,500-residue proteins (rows 6–9) are shown in black and blue characters, respectively. Identical residues within sequences of the first and second groups are highlighted in green and blue, respectively. Residues that are identical in all or most sequences are highlighted in red and purple, respectively. Secondary-structure elements derived from the structure of the IpaH1.4 C-terminal domain are shown above the sequence alignment and labeled. The broken lines correspond to the disordered regions in the structure. The α-helices that are part of N-terminal, middle and C-terminal lobes are colored blue, red and cyan, respectively. Residues selected for mutagenesis are marked with asterisks.