Abstract
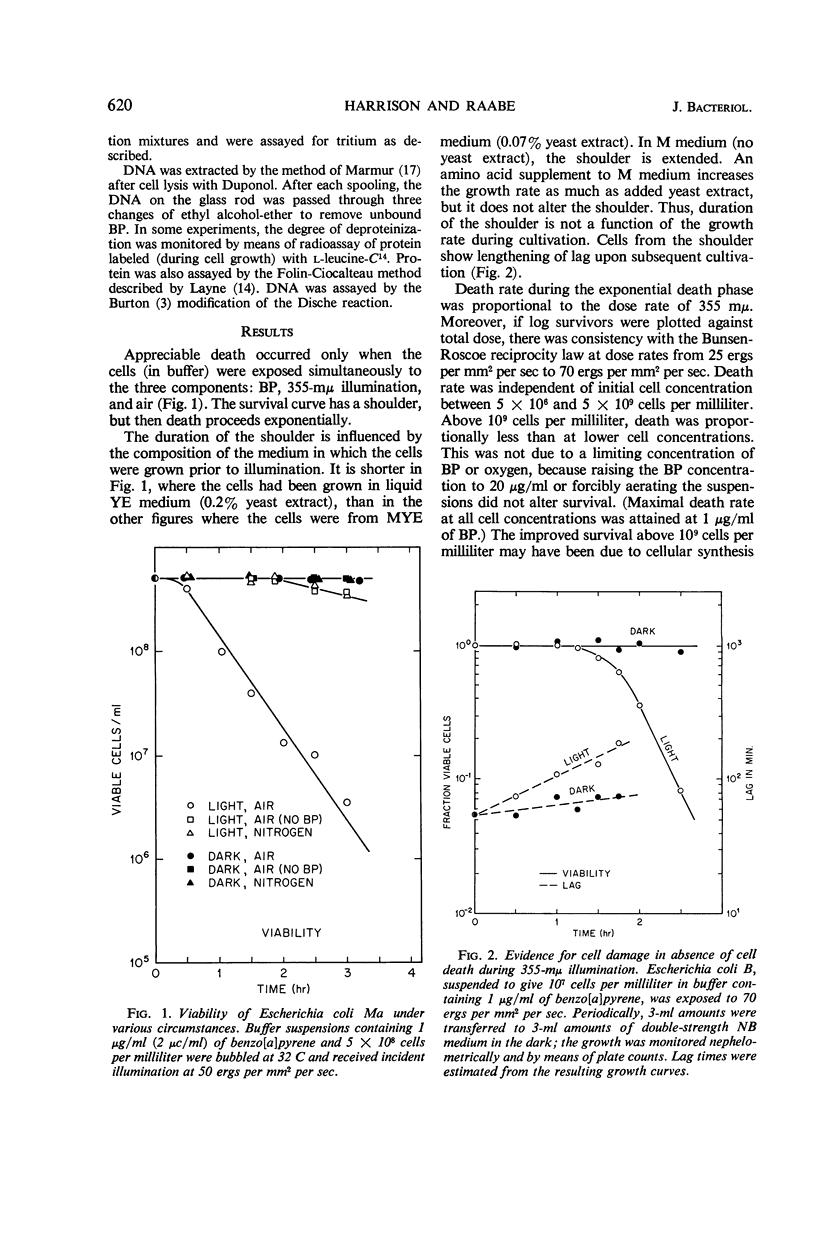
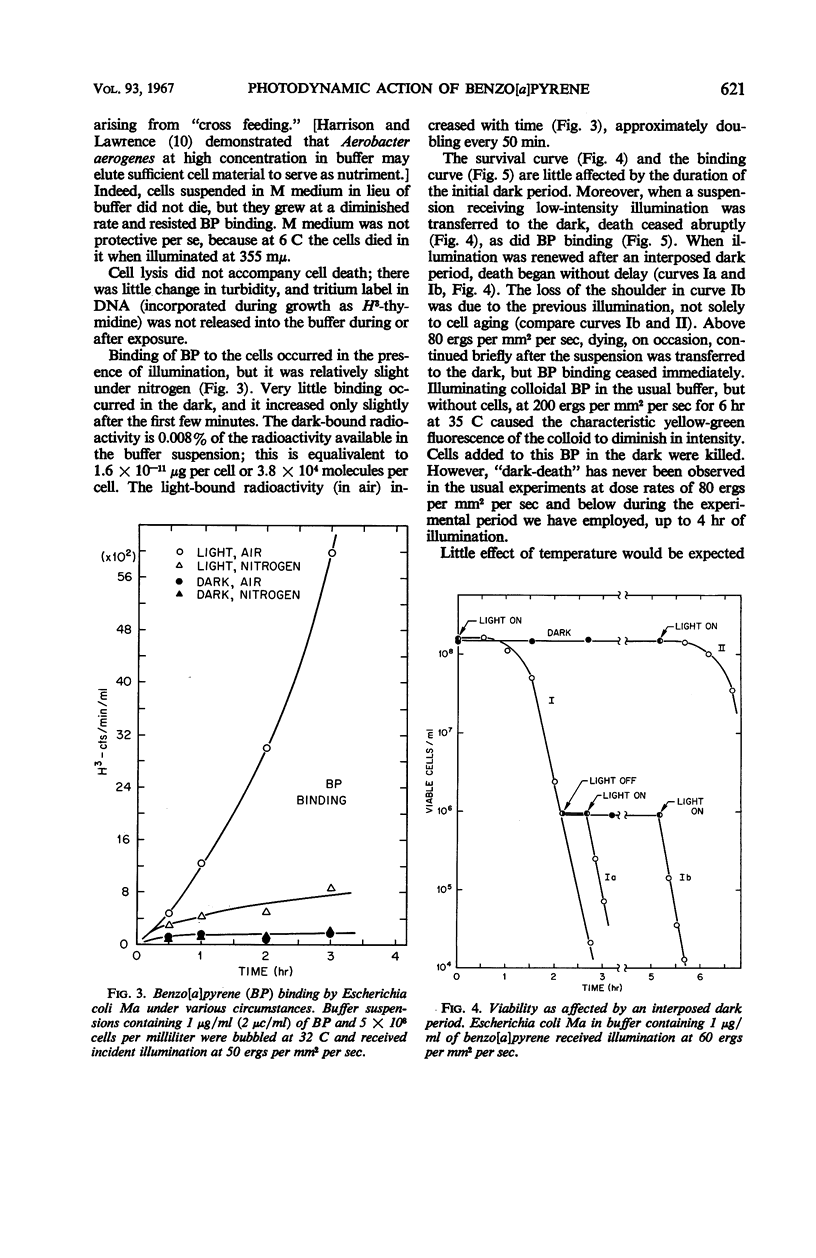
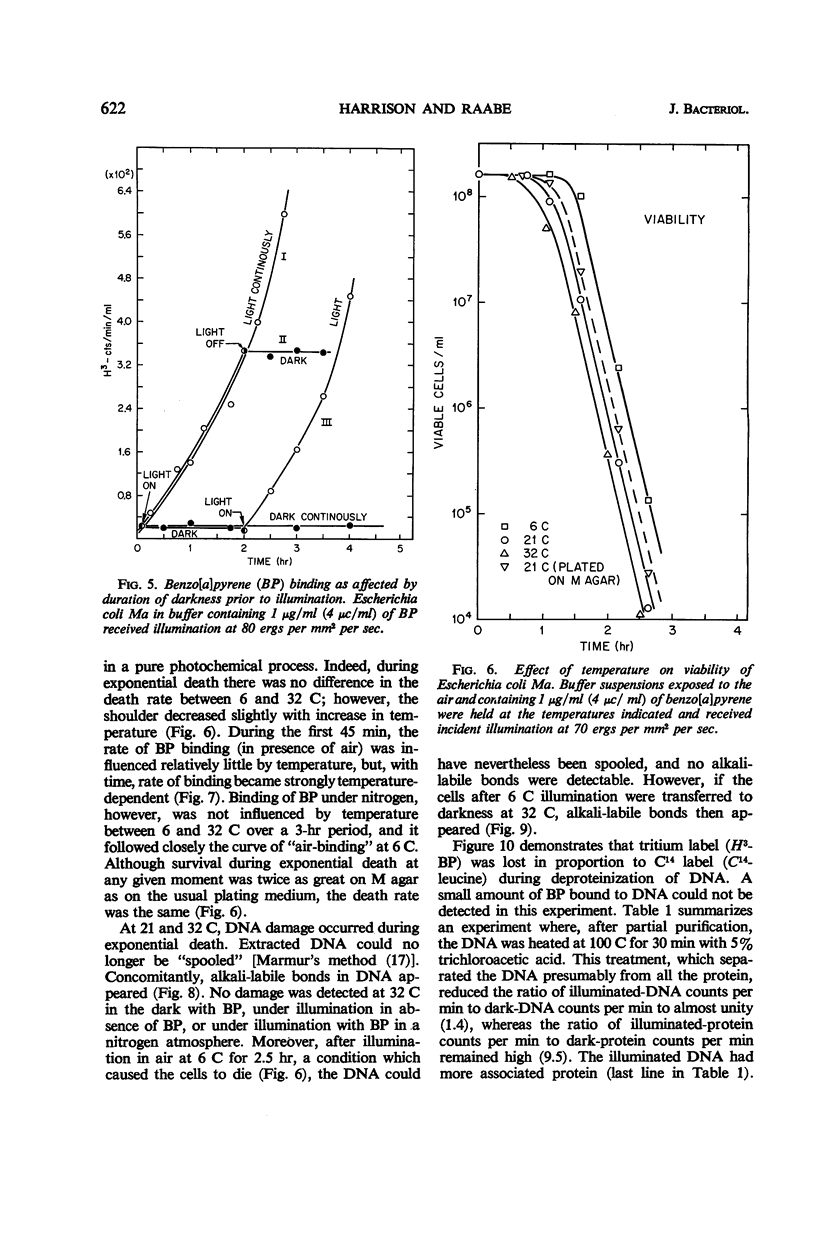
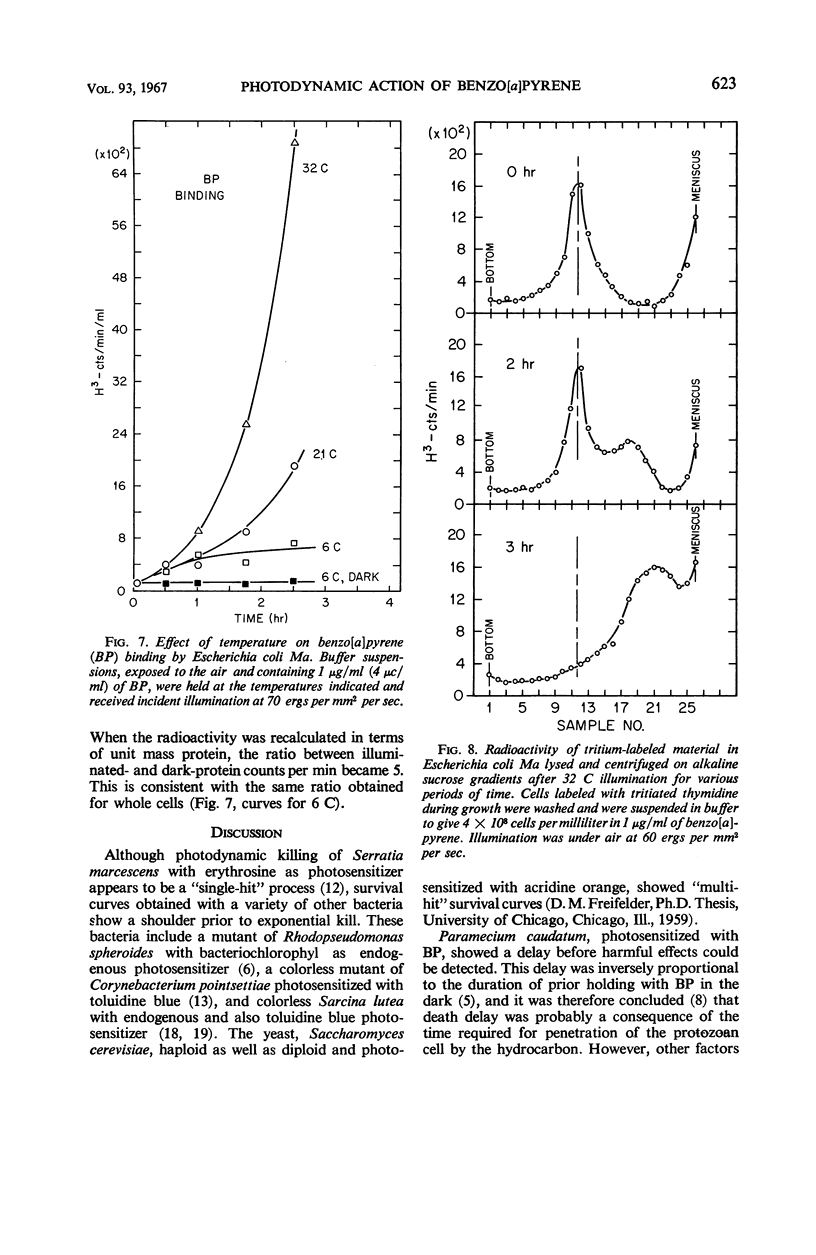
Death of Escherichia coli resulted when a buffer suspension was exposed simultaneously to colloidal benzo[a]pyrene (BP) and 355-mμ illumination. Neither hydrocarbon nor illumination alone caused death; oxygen had to be present. The survival curve had a shoulder, and then death proceeded exponentially with time. Death rate was independent of temperature between 6 and 32 C. The duration of the shoulder, however, decreased slightly with increase in temperature. The shoulder was not due to delay in BP entering the cell. Death was influenced by the composition of the medium in which the cells were grown prior to illumination. The amount of BP bound to the cells was determined after three ethyl alcoholether extractions. Appreciable binding occurred in the presence of 355-mμ illumination with air, and relatively little binding occurred under nitrogen; very little binding occurred in the dark with nitrogen or air. At the outset, rate of binding under illumination with air was not temperature-dependent, but with time it became strongly temperature-dependent. Binding under illumination with nitrogen was temperature-independent. Bound BP was associated primarily with cell protein. Cells in growth medium resisted death and BP binding. At 21 and 32 C, deoxyribonucleic acid damage occurred during exponential death. No damage was detected at 21 and 32 C in the dark with BP, under illumination in absence of BP, or under illumination with BP in a nitrogen atmosphere.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., HARDIGREE A. A. POSTIRRADIATION GROWTH, DIVISION, AND RECOVERY IN BACTERIA. Radiat Res. 1965 May;25:92–102. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchard R. P., Dworkin M. Light-induced lysis and carotenogenesis in Myxococcus xanthus. J Bacteriol. 1966 Feb;91(2):535–545. doi: 10.1128/jb.91.2.535-545.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORKIN M. Endogenous photosensitization in a carotenoidless mutant of Rhodopseudomonas speroides. J Gen Physiol. 1958 Jul 20;41(6):1099–1112. doi: 10.1085/jgp.41.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORKIN M. Function of carotenoids in photosynthetic bacteria. Nature. 1959 Dec 12;184(Suppl 24):1891–1892. doi: 10.1038/1841891b0. [DOI] [PubMed] [Google Scholar]
- HARRISON A. P., Jr, LAWRENCE F. R. PHENOTYPIC, GENOTYPIC, AND CHEMICAL CHANGES IN STARVING POPULATIONS OF AEROBACTER AEROGENES. J Bacteriol. 1963 Apr;85:742–750. doi: 10.1128/jb.85.4.742-750.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A. P., Jr Thymine incorporation and metabolism by various classes of thymine-less bacteria. J Gen Microbiol. 1965 Dec;41(3):321–333. doi: 10.1099/00221287-41-3-321. [DOI] [PubMed] [Google Scholar]
- JAGGER J. A small and inexpensive ultraviolet dose-rate meter useful in biological experiements. Radiat Res. 1961 Apr;14:394–403. [PubMed] [Google Scholar]
- KAPLAN R. W. Dose-effect curves of s-mutation and killing in Serratia marcescens. Arch Mikrobiol. 1956;24(1):60–79. doi: 10.1007/BF00418422. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUBIN M. Enrichment of auxotrophic mutant populations by recycling. J Bacteriol. 1962 Mar;83:696–697. doi: 10.1128/jb.83.3.696-697.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHEWS M. M., SISTROM W. R. Function of carotenoid pigments in non-photosynthetic bacteria. Nature. 1959 Dec 12;184(Suppl 24):1892–1893. doi: 10.1038/1841892a0. [DOI] [PubMed] [Google Scholar]
- MATHEWS M. M., SISTROM W. R. The function of the carotenoid pigments of Sarcina lutea. Arch Mikrobiol. 1960;35:139–146. doi: 10.1007/BF00425002. [DOI] [PubMed] [Google Scholar]
- Moore B. G., Harrison A. P., Jr Benzo[a]pyrene uptake by bacteria and yeast. J Bacteriol. 1965 Oct;90(4):989–1000. doi: 10.1128/jb.90.4.989-1000.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA T., HOMMA J., SONOHARA H. Improved method for obtaining thymineless mutants of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1962 Sep;84:602–603. doi: 10.1128/jb.84.3.602-603.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATRICK M. H., HAYNES R. H., URETZ R. B. DARK RECOVERY PHENOMENA IN YEAST. 1. COMPARATIVE EFFECTS WITH VARIOUS INACTIVATING AGENTS. Radiat Res. 1964 Jan;21:144–163. [PubMed] [Google Scholar]
- TSO P. O., LU P. INTERACTION OF NUCLEIC ACIDS, II. CHEMICAL LINKAGE OF THE CARCINOGEN 3,4-BENZPYRENE TO DNA INDUCED BY PHOTORADIATION. Proc Natl Acad Sci U S A. 1964 Feb;51:272–280. doi: 10.1073/pnas.51.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]


