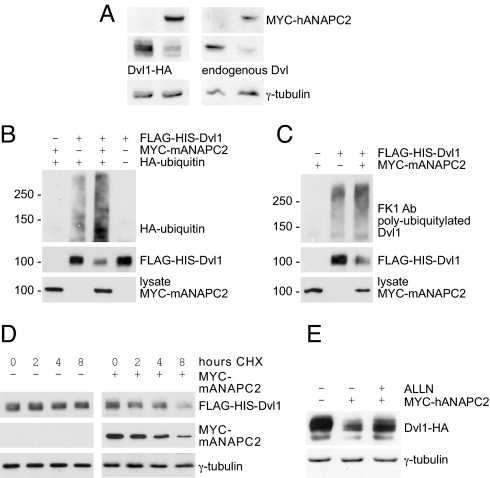
Fig. 1.
ANAPC2 targets Dvl for ubiquitin-dependent degradation. (A) Levels of both transiently expressed (Left) and endogenous Dvl (Right) were reduced in HEK 293T cells expressing ANAPC2; γ-tubulin levels were used as a loading control. (B) Expression of Myc-tagged ANAPC2 led to an increase in ubiquitiylated Dvl in HEK 293T cells, cotransfected with Flag-His-tagged Dvl1 and HA-tagged ubiquitin. (C) Coexpression with Myc-ANAPC2 enhanced the conjugation of endogenous ubiquitin to Dvl1, as detected by the polyubiquitin antibody FK1. (D) The half-life of Dvl1 decreased in the presence of ANAPC2. Forty-eight hours after the transfection of HEK 293T cells, 40 μg·mL −1 cycloheximide (CHX) was added for 0, 2, 4, and 8 h. The levels of Dvl1 and Myc-ANAPC2 were monitored by Western blot analysis, and γ-tubulin levels were used as a loading control. (E) The proteasome inhibitor ALLN (30 μM) reversed the ANAPC2-mediated reduction of Dvl1 levels; γ-tubulin levels were used as a loading control.