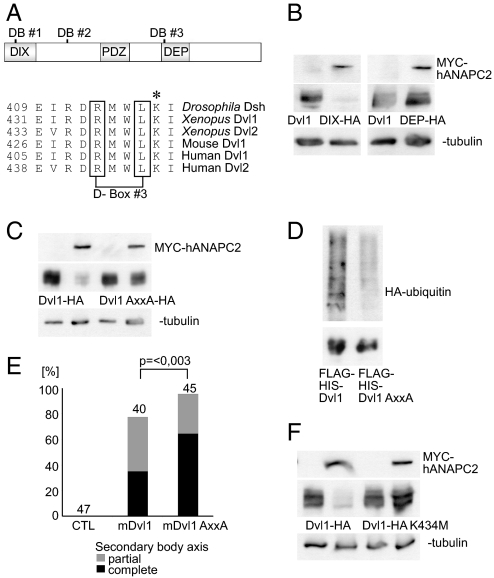
Fig. 4.
Degradation of Dvl by ANAPC2 depends on a conserved D-box within the DEP domain. (A) Schematic representation of mouse Dvl1 indicating the three potential D-boxes and the DIX, PDZ, and DEP domains of the protein. Alignment of the amino acid sequence of Drosophila, Xenopus, mouse, and human Dvl flanking the third D-box. The asterisk (*) denotes a conserved lysine residue immediately adjacent to the third D-box. (B) APC2 reduced the protein levels of a mutant mouse Dvl1 that lacks the first two D-boxes (Dvl1ΔDIX-HA) but had no effect on a mouse Dvl1 construct missing the third D-box (Dvl1ΔDEP-HA). (C) Mutation of D-box 3, replacing the RxxL motif by AxxA, protected Dvl1 against the effects of ANAPC2; γ-tubulin served as a loading control. (D) Ubiquitylation of a Flag-His-tagged Dvl1 mutant (Flag-His-Dvl1 AxxA) is reduced compared with Flag His-tagged wild-type Dvl1 (Flag-His-Dvl1). With a nickel column, the His-tagged proteins were isolated under denaturing conditions from transiently transfected HEK 293T cells coexpressing HA-tagged ubiquitin. (Upper) Incorporation of HA-ubiquitin. (Lower) Demonstration of the precipitated Dvl1 proteins, using an anti-Flag antibody. (E) As a consequence of its increased stability, mouse Dvl1 AxxA induces more secondary body axes in Xenopus embryos compared with the wild-type protein (P < 0.003, Mann–Whitney rank sum test). (F) The lysine at position 434 in mouse Dvl1 is critical for ANAPC2-dependent degradation of Dvl1 levels. In transfected HEK 293T cells, a Dvl1 K434M mutation is not targeted by ANAPC2.