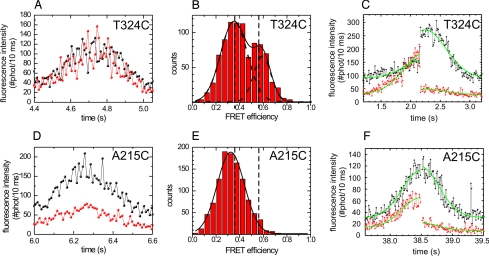
Fig. 2.
FRET is observed for donor—acceptor-labeled T324C kinesin and not for A215C. ATP concentration: 2 mM, unless stated differently (C and F). (A) T324C fluorescence intensity time trace binned over 10 ms showing large fluctuations. Black, donor fluorescence intensity; red, acceptor fluorescence intensity. (B) Apparent-FRET-efficiency histogram of T324C [IA/(IA + ID), binned over 2 ms, central 200 ms of 6 events (other events were not taken into account for this analysis, because they were affected by landing or photo bleaching within this time interval)] showing two peaks (at FRET efficiencies of 0.353 ± 0.009 and 0.59 ± 0.01, obtained from double Gaussian fit). (C) Intensity time trace of a single T324C event. The green lines represent global fits with two Gaussians (one for the donor and one for the acceptor channel) with a common width, offset, and centre position and with an amplitude step at the moment of acceptor photo-bleaching. The fitted amplitudes show that after acceptor photo bleaching (at ≈2.2s) the donor intensity increased by a factor of 2.6 ± 0.2. For this trace the ATP concentration was 20 μM. (D) A215C fluorescence intensity time trace. The intensity fluctuations appear lower than in A. (E) Apparent-FRET-efficiency histogram of A215C (5 events). Only one peak, corresponding to the low FRET one in B, is observed (FRET efficiency = 0.325 ± 0.003). (F) Intensity time trace of a single A215C event. After acceptor photo bleaching (at ≈38.5 s), the donor intensity is hardly affected (increase of a factor 1.07 ± 0.05). For this trace, the ATP concentration was 50 μM.