Abstract
Rationale:
Protein Kinase C (PKC) regulates contractility of cardiac muscle cells by phosphorylating thin- and thick- filament based proteins. Myocardial sarcomeres also contain a third myofilament, titin, and it is unknown whether titin can be phosphorylated by PKC and if it affects passive tension.
Objective:
Study the effect of PKC on titin phosphorylation and titin-based passive tension.
Methods and Results:
Phosphorylation assays with PKCα revealed that titin is phosphorylated in skinned myocardial tissues; this effect is exacerbated by pre-treating with PP1. In vitro phosphorylation of recombinant protein representing titin's spring elements showed that PKCα targets the PEVK spring element. Furthermore, mass spectrometry in combination with site-directed mutagenesis identified two highly conserved sites in the PEVK region that are phosphorylated by PKCα (S11878 and S12022); when these two sites are mutated to alanine, phosphorylation is effectively abolished. Mechanical experiments with skinned LV myocardium revealed that PKCα significantly increases titin-based passive tension, an effect that is reversed by PP1. Single molecule force-extension curves show that PKCα decreases the PEVK persistence length (from 1.20 nm to 0.55 nm), without altering the contour length, and using a serially-linked wormlike chain (WLC) model we show that this increases titin-based passive force with a sarcomere length dependence that is similar to that measured in skinned myocardium following PKCα phosphorylation.
Conclusions:
PKC phosphorylation of titin is a novel and conserved pathway that links myocardial signaling and myocardial stiffness.
Keywords: connectin, diastole, passive stiffness, post-translational modification
Introduction
Post-translational modifications of contractile and regulatory proteins profoundly alter myocardial function during normal physiological adaptations as well as during pathological processes. Two key mediators of many diverse physiological and pathological responses are the β-adrenergic pathway that results in activation of Protein Kinase A (PKA) and the α1-adrenergic pathway that results in activation of Protein Kinase C (PKC). Many cardiac proteins can be phosphorylated by both PKA and PKC and, interestingly, this can have either similar or disparate effects, with interplay among the phosphorylation sites (for recent review with original citations, see1). In this study we focused on phosphorylation of the giant protein titin.
Titin, the largest protein known, is the third most abundant myofilament of striated muscle (after myosin and actin) and spans the half sarcomeric distance from Z-disk to M-line2. In the I-band region of the sarcomere titin is extensible and functions as a molecular spring that develops force in sarcomeres stretched beyond their slack length (~1.9 μm). This force largely determines the passive tension of the cardiac myocyte and, together with collagen, determines myocardial passive stiffness3.
Titin's spring region is comprised of three highly modular sequence motifs: the serially-linked immunoglobulin(Ig) -like domains, the N2B element, and the PEVK region (so named because it contains primarily proline (P), glutamate (E), valine (V) and lysine (K) residues)2. Previous phosphorylation studies have focused on the cardiac-specific N2B element, and identified that this element can be phosporylated by PKA4, and by Protein Kinase G (PKG)5. Both PKA and PKG phosphorylation increased titin's compliance (reduced stiffness)4-6, with hypophosphorylation of titin in heart failure giving rise to reduced compliance7, indicating the importance of titin phosphorylation in normal physiological adaptations as well as in pathophysiological processes.
In this work we focused on whether PKC phosphorylates titin. PKC is a group of closely related serine-threonine signal transduction kinases that are involved in many cellular processes including regulation of cytosolic [Ca2+], myofilament Ca2+ sensitivity, and contractility of cardiac muscle cells1. PKC family members have also been implicated in cardiovascular pathology, including ischemic heart disease, congestive heart failure, myocardial hypertrophy and hypertension8. In addition, several studies have shown that the activity and expression of numerous PKC isoforms is upregulated in experimental models and human heart failure (9 and references therein).
Experiments were performed with a constitutively active PKC fragment and with PKCα, a key member of the PKC family, involved in the development of contractile dysfunction in heart failure10. We found that titin is phosphorylated by PKC and that Protein Phosphatase 1 (PP1) pre-treatment significantly increases subsequent PKC phosphorylation. In vitro kinase assays showed that the PEVK region of titin is a PKC substrate. Furthermore, mass spectrometry and site-directed mutagenesis identified two sites in the PEVK region that are phosphorylated by PKC. Mechanical studies showed that PKC phosphorylation increases titin-based tension and that the effect is amplified by PP1 pre-treatment. Consistent with these findings, single molecule studies showed that PKC phosphorylation reduces the persistence length (PL) of the PEVK. Thus, the PEVK region of titin is a prominent site of PKC phosphorylation, and PKC-based phosphorylation increases passive tension.
Materials and Methods (details in the supplement)
Skinned muscle phosphorylation
Mouse and porcine LV muscles were used. A constitutively active PKC fragment (PKCcf) and a calcium and phosphatidylserine dependent PKCα were used with 20 μCi of [γ-32P]ATP. Samples were electophoresed on 2-7% gel gradient gels that were stained, dried, scanned, and exposed to X-ray film and analyzed. The titin integrated OD of the autoradiograph was normalized to that of the Coomassie blue-stained gel, to normalize for protein loading. All experiments were approved by the University of Arizona IACUC and followed the U.S. National Institutes of Health “Using Animals in Intramural Research” guidelines for animal use.
In vitro kinase assay
The recombinant titin fragments N2B, PEVK (N2B cardiac isoform) and Ig1-8 (see Fig. 2A) were expressed in E. coli as described11-12 with site directed mutagenesis as in Labeit et al.13 (See also Supplement.) Fragments were incubated with PKCα and [γ-32P]ATP. The proteins were electrophoresed and gels (4-20%) were stained, dried, scanned, exposed to X-ray film and analyzed as described above.
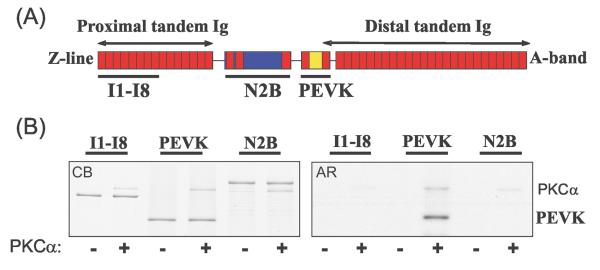
Figure 2. Identification of the PKC target domain in extensible region of N2B isoform of cardiac titin.
A) Schematic of titin's I-band region showing location of recombinant I1-8, N2B and PEVK elements that were studied. (B) In vitro kinase assay with PKCα. Only the PEVK element is a PKCα substrate. Left panel: Coomassie blue stained gel (CB); right panel: corresponding autoradiograph (AR). Note autophosphorylation of PKCα.
Measurement of passive tension
Mouse and porcine muscle strips were actin extracted with gelsolin and either first treated with PP1 or directly studied. Passive tension, fiber length and sarcomere length (SL) were measured as previously described14. Relaxed fibers were slowly stretched (10%/sec) from their slack length to a SL of 2.3μm, followed by a 90 second hold. (Selected because stress relaxation had markedly slowed down at this time.) We then imposed a frequency sweep (FS; 1-70 Hz, amplitude 1%) and measured complex stiffness (E) and the phase shift (δ) between force and length. Elastic and viscous moduli were determined as E (cos δ) and E (sin δ), respectively. Finally, the fiber was released back to its slack length. Fibers were then treated with PKCα, followed by stretch-hold-FS protocols. In other experiments the effect of PP1 on passive tension was measured. We first dephosphorylated with PP1 (dephosphorylating PKC sites and possibly other sites), and then we phosphorylated with PKCα. The preparations were then mounted in the mechanical setup and passive tension was measured before and after a second PPI treatment (now dephosphorylating only PKC sites). To determine titin and collagen contribution to passive force, thick and thin filaments were extracted, removing titin's anchors in the sarcomere3. The remaining force, assumed to be collagen based, was subtracted from the pre-extraction forces to determine titin-specific forces (see3).
Single molecule force spectroscopy
PEVK molecules (with the flanking Ig domains, Ig27 and Ig84) were stretched using an atomic force microscope as previously described12. For analysis we used the worm-like chain equation (WLC)15: F·PL/kBT = z/CL+(1/4(1-z/CL)2)-1/4 (equation 1); F is force (pN), PL is the persistence length (nm), z is end-to-end extension (nm), CL is the contour length (nm), kB is Boltzmann's constant, and T is absolute temperature. Records in which 2 regularly-spaced Ig-unfolding peaks appear in the force trace were analyzed, ensuring that the full PEVK had been stretched. The initial trace of the force-extension curve (Fig. 4A, right) was fit with the WLC model and PL and CL of the PEVK were determined. In addition, the force-extension curves between the 1st and 2nd peaks were fit. The effects of changes in PEVK PL following PKC phosphorylation on titin-based force-SL curves were calculated by using the WLC equation and serially-linking the three WLCs (the combined tandem Ig segments, the PEVK, and N2B-Us elements). For details see16.
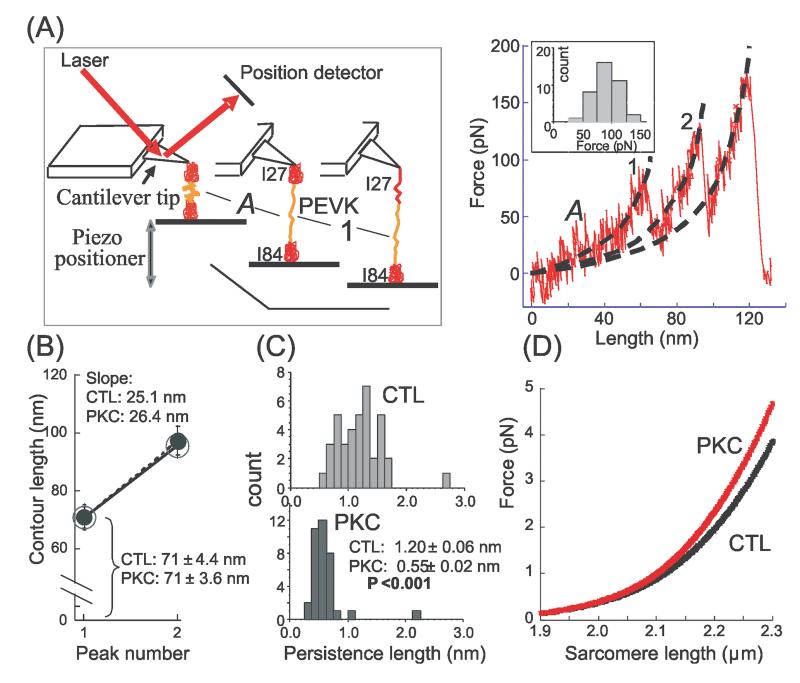
Figure 4. Effect of PKCα phosphorylation on single PEVK molecule mechanics.
A) Left: Schematic of atomic force microscope (AFM) experiment with a construct containing the PEVK and its flanking Ig domains (Ig27 and Ig84); Right: representative force-extension curve. Stage ‘A’ refers to PEVK extension and ‘1’ to unfolding of the first Ig domain (either Ig27 or Ig84). Subsequently, the second Ig domain unfolds giving rise to a second force peak; the third peak is due to detachment of the molecule. Dashed black lines represent WLC fits that were used to determine the contour length (CL) and persistence length (PL) with values obtained for stage ‘A’ representing the PEVK. Inset shows the unfolding force histogram for the first unfolding peak (mean value 90 ± 3 pN). B) CL of PEVK (peak 1) and PEVK plus one unfolded Ig domain (peak 2). Nonphosphorylated (CTL): large open circle (n = 38); phosphorylated (PKC): small closed circle (n = 36). Phosphorylation has no effect on CLs. The shown slopes represent the contour-length-gain due to Ig domain unfolding; slopes are unaffected by PKC. C) PEVK PL histograms before (CTL, top) and after (PKC, bottom) PKC phosphorylation. Distribution is shifted to lower values following phosphorylation; mean PL value is significantly reduced. D) Calculated effect of PEVK PL reduction from 1.2 to 0.55 nm on force-sarcomere length curve of a single titin molecule (cardiac N2B isoform).
Tandem mass spectrometry coupled to liquid chromatography (LC-MS/MS)
Gels were loaded with nonphosphorylated and PKCα-phosphorylated PEVK and were Coomassie blue stained. The PEVK bands were excised from the gels and digested with trypsin. LC-MS/MS analyses were carried out and tandem MS spectra of peptides were analyzed with TurboSEQUEST™. Possible modifications such as alkylation of cysteine residues and oxidation of methionine residues as well as other possible modifications (e.g., +80 Da due to phosphorylation) were included in the search parameters.
Statistical Analysis
Data are presented as mean ± SE. Significant differences were probed using ANOVA. Post hoc comparisons were made using Bonferroni. Probability values <0.05 were taken as significant.
Results
Titin phosphorylation by PKCcf and PKCα
To determine whether titin in mouse LV skinned myocytes can be phosphorylated by PKC we performed back-phosphorylation assays with a constitutively active PKC fragment (PKCcf) prepared from a mixture of α, β, and γ PKC isoforms (BIOMOL International). In addition to well-known PKC substrates (such as MyBP-C), the autoradiograph shows phosphorylation of titin (Figure 1A). Both the intact titin molecule (the dominant isoform in mouse myocardium is the N2B isoform) and the degradation product (T2) are phosphorylated. To further investigate the role of PKC in titin phosphorylation we used the full-length PKCα isoform, which is calcium and diacylglycerol/phosphatidylserine dependent (we selected PKCα because of its prominent role in depressing contractile function in heart failure9). Clear phosphorylation in titin can be seen with phosphorylation occurring primarily within N2B titin (Figure 1B). We also used PKCα on skinned LV mouse myocardium (Figure 1C). Similarly to mouse myocytes, PKCα induced phosphorylation primarily within N2B titin. The low T2 phosphorylation following PKCα treatment suggests that PKCα's phosphorylation site(s) lie outside the A-band region of titin (which primarily makes up T2 titin).
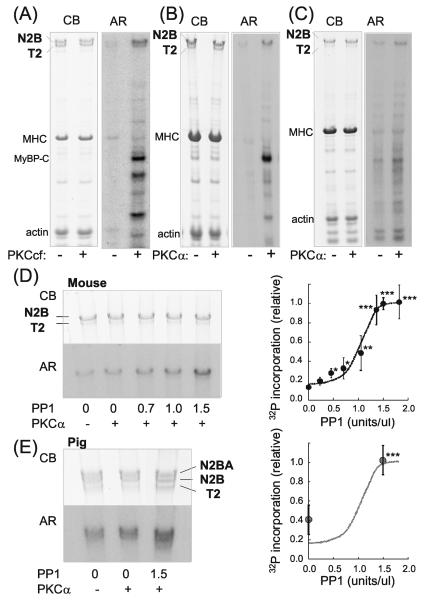
Figure 1. Cardiac titin is phosphorylated by PKCα and dephosphorylated by PP1.
A-C) Representative results on mouse skinned cardiac myocytes (A and B) and skinned myocardium (C) incubated in [γ-32P]ATP without (−) or with (+) PKC. Left panels: Coomassie blue (CB) stained gel and right panels: corresponding autoradiograph (AR). In A) the catalytically active PKC fragment (PKCcf) was used and in B) and C), PKCα. In addition to the well-known PKC substrates MyBP-C and TnI, titin is also phosphorylated. The majority of titin is T1 (upper band representing full-length titin) and a small fraction is T2 (degradation product containing the A-band region of titin). T1 is phosphorylated by both PKCcf and PKCα and T2 only by PKCcf. D and E) Effect of pre-treating skinned myocardium with PP1 on PKCα phosphorylation in mouse (D) and porcine LV (E). 32P incorporation is enhanced by PP1, indicating that PP1 dephosphorylates sites on titin that function subsequently as PKC substrates. Note that pig myocardium coexpresses N2B and N2BA cardiac titin and that both isoforms are phosphorylated by PKCα and dephosphorylated by PP1. Graphs show 32P incorporation relative to the incorporation following pretreatment with 1.5 PP1 U/μl, and normalized by protein loading. The graph in D) reveals that a maximal PP1 effect (n = 5) is seen at ~1.5 U/μl PP1 and E) indicates that at 0 U/μl PP1 pig myocardium (n = 3) is phosphorylated to 40% of maximal, a value several-fold larger than for the mouse (18%). This indicates a higher basal phosphorylation in mouse myocardium than in pig. *significant vs prePKCα. Broken line is the fit to the mouse data of Fig. 1D.
In order to determine the basal phosphorylation level within murine LV myocardium, the skinned tissues were preincubated with increasing concentrations of PP1 to release phosphate groups from phosphorylated residues on titin (Figure 1D). (Note that the back phosphorylation assay only shows 32P added during PKC incubation and not phosphorylated sites already present.) The removal of basal phosphate groups increased the availability of PKCα sites in a concentration dependent manner with maximum dephosphorylation occurring at PP1 concentrations of ~1.5U/μl and an EC50 of ~1.0 U/μl (Figure 1D, right inset). To determine whether basal phosphorylation levels differ between large and small mammals we also studied skinned porcine LV myocardial tissue (Figure 1E). Incubation of pig myocardium, which coexpresses N2B and N2BA cardiac titin isoforms, with PKCα showed that both titin isoforms are phosphorylated by PKCα. Furthermore, preincubation with PP1 increased the 32P incorporation by PKCα. Interestingly, PKCα phosphorylated porcine myocardium without preincubation of PP1 to a higher degree (~40%) (Figure 1E, right) relative to murine myocardium (~20%) (Figure 1D, right). Conversely, PP1 pretreatment had a greater effect, as compared to PKCα alone, on murine myocardium than on porcine myocardium. These results indicate a higher basal phosphorylation level in mouse LV myocardium than in pig LV myocardium, estimated at 82 and 60%, respectively. In summary, titin is a PKC substrate with PKCα phosphorylating predominately the full-length titin molecule.
In vitro phosphorylation of recombinant titin fragments
The finding that PKCα preferentially phosphorylates T1 suggests that the phosphorylation site(s) lie(s) within the spring elements that make up titin's elastic I-band region. Hence we performed in vitro PKCα phosphorylation assays with recombinant proteins that represent the three spring elements found in the N2B cardiac titin isoform: the PEVK element including its flanking Ig domains Ig27 and Ig84, the N2B element and its flanking Ig domains, and Ig1-8 as a representative of the tandem Ig segments (Figure 2A). PKCα phosphorylated the PEVK element but not the N2B element or the Ig1-8 (Fig. 2B). Because the PEVK element of the N2B cardiac isoform is constitutively expressed in all titin isoforms, PKCα phosphorylation can therefore be expected to take place universally (this is consistent with our finding that both N2BA and N2B titin is phosphorylated in porcine myocardium). Concurrently, this finding suggests that titin phosphorylation by PKCα could play a role in modulating passive tension development because the PEVK domain is a source of titin extensibility within the physiological sarcomere length range and becomes progressively more important at SLs>~2.1 μm17.
Effect of PKCα on passive tension
To study the effect of PKCα on passive tension we used skinned LV myocardium that had been actin-extracted with gelsolin, to abolish actomyosin interaction and ensure that PKCα effects are solely due to the passive properties of the preparations. Following gelsolin extraction some preparations were directly studied, others were first treated with PP1. The preparations were stretched to a sarcomere length of 2.3 μm and held for 90 s, followed by a sinusoidal stiffness analysis, and a release back to their slack length. This protocol was repeated following PKC incubation.
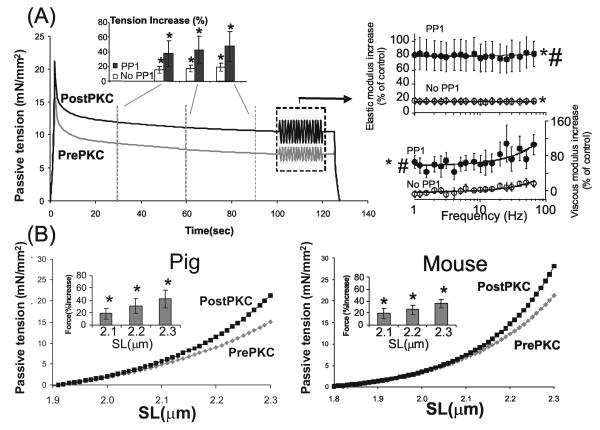
PKCα treatment significantly increased passive tension during the hold phase by ~15-20%, an effect exacerbated by PP1 pretreatment (Fig.3). To examine whether the passive tension increase can be reversed, we measured tension before and after PP1 treatment (See Methods) and found that PP1 reduced passive tension (Fig. S1). PKCα treatment significantly increased the elastic moduli, an effect significantly larger following PP1 pre-treatment (Figure 3A, right inset). The viscous moduli were only significantly increased with PP1 pretreatment (Figure 3A, right). The mean increase in the noPP1 experiments was ~15% for the elastic moduli and ~5% for the viscous moduli. This difference indicates that the mechanisms underlying the elastic and viscous responses have a different dependence on the number of phosphorylated titin molecules.
Figure 3. Effect of PKCα phosphorylation on titin-based passive tension in skinned myocardium.
(A) Passive tension during stretch-hold-release protocol before PKCα (prePKC) and after incubation with PKCα (postPKC) in porcine LV skinned myocardium (mean values of 6 fibers). The preparations had been pre-treated with gelsolin to extract thin filaments and prevent actomyosin interaction during and after PKCα treatment. Fibers were preincubated with PP1 to dephosphorylate titin before PKCα incubation. Fibers were stretched from their slack length (SL ~ 1.9 μm) to a SL of 2.3 μm and were then held, followed by a release back to the slack length. Passive tension is significantly higher following PKCα-treatment. Towards the end of the hold phase a frequency sweep was imposed (1-70 Hz, amplitude 1%) (frequency sweep shown is idealized) and elastic and viscous moduli determined. Right: Percent increase in elastic and viscous moduli from pre-PKCα controls, with or without PP1 pre-treatment. Elastic and viscous moduli are higher following PKCα treatment, with PP1 pre-treatment enhancing the effect. (B) Titin-based passive tension-SL relationship measured during stretch of porcine fibers (left panel; mean of 6 muscles) and mouse fibers (right panel; mean of 6 muscles) that had been gelsolin extracted and pre-treated with PP1. The shown mean values indicate that PKCα treatment significantly increases titin-based tension; the increase is small at SLs below ~2.1 μm but progressively increase at longer SLs. Insets show the percent increase in tension at three SLs. * significant vs prePKCα (P<0.05); # Significant vs No PP1.
We also determined the SL dependence of the PKC effect on titin-based passive tension both for porcine (Fig. 3B, left) and mouse skinned myocardium (Fig. 3B, right). These experiments were performed on gelsolin-extracted and PP1 pre-treated skinned preparations. In both mouse and pig, titin-based passive tension was higher after PKCα treatment and the effect increased with SL at lengths longer than ~2.1μm, indicating PEVK's involvement in PKCα-induced increases in passive tension.
Single Molecule force spectroscopy
To gain insights into the mechanism of increased passive tension we performed single molecule force spectroscopy on the PEVK (the cardiac N2B isoform) with its flanking Ig domains, Ig27 and Ig84. The force extension curve of the construct was measured with an atomic force microscope (AFM) as explained in Fig. 4A, left with a typical example shown in Fig. 4A, right. The abrupt force reductions following the force peaks are likely due to unfolding of Ig domains that lengthen the molecule (from ~5 nm to ~30 nm). Up to three peaks were seen, consistent with 2 Ig unfolding events and a final peak due to detachment of the molecule from its anchoring point(s). The initial trace leading up to the first unfolding peak (‘A’ in Fig 4A) corresponds to the force-extension curve of the PEVK. We fit this initial trace with the WLC model (see Methods) and determined the persistence length (PL) and the contour length (CL) of the PEVK; we also fit the traces leading up to the second unfolding peak. Results from molecules that had been phosphorylated by PKCα and those that had not (CTL) were compared.
Fig. 4B shows that the CL of the PEVK is ~70 nm, in line with previous work11, 18, and that CL is insensitive to PKCα phosphorylation. The CL gain that results from Ig domain unfolding (slope of line in Fig. 4B) is also unaffected by PKCα. The PL of the nonphosphorylated PEVK (Fig. 4C top) had a broad distribution, similar to that described earlier18. Following phosphorylation, the PEVK PL distribution was narrower and shifted to shorter lengths; the mean PL value was significantly lower than for the control molecules (Fig. 4C, bottom). We evaluated the effect of the PL reduction on force developed by a single titin molecule (N2B isoform) as a function of SL, using a serially-linked WLC model of titin's extensible region in the sarcomere (see Supplement). Results show that titin's force is increased in a SL-dependent manner. The effect is small at SLs< ~ 2.1 μm but increases at longer SLs, and reaches 22.5% at SL 2.3 μm.
Localization of the PEVK phosphorylation site(s)
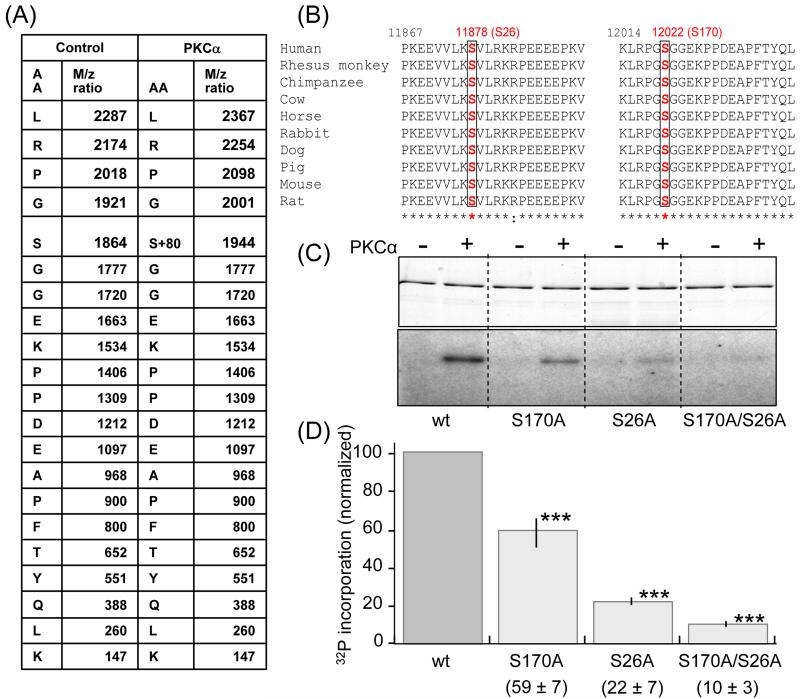
To identify the amino acid residue(s) phosphorylated by PKCα in the PEVK element we used in-gel tryptic digestion and nano LC-MS/MS analysis on the recombinant titin PEVK fragment (including flanking Ig domains) with or without PKCα phosphorylation. The tryptic digestion produced 53 fragments that were separated by LC and analyzed by tandem MS. Mass spectrometry detected only a single tryptic fragment that was phosphorylated by PKCα (LRPGSGGEKPPDEAPFTYQLK). The fragmentation table of this tryptic fragment, shown in Fig. 5A, reveals that the serine contained in this fragment (S12022 in full length human titin (Swiss ProtQ8wz42) or S170 in PEVK of cardiac N2B titin isoform) is phosphorylated. The identified fragment including its serine is fully conserved among the wide range of mammalian species whose PEVK sequence is known (Figure 5B right).
Figure 5. Identification of target PKCα phosphorylation sites on PEVK.
Mass spectrometry was carried out on nonphosphorylated (control) and PKCα phosphorylated PEVK (plus flanking Ig domains). Samples were electrophoresed, followed by in-gel tryptic digestion and analysis by MS/MS. A) Of the 53 identified digestion fragments only fragment LRPGSGGEKPPDEAPFTYQLK had a differential mass of 80 × n Dalton; its fragmentation table with its amino acid sequence and the m/z ratio are shown. The control fragment has a molecular mass of 1863.9 dalton and the phosphorylated fragment 1943.9 dalton (1863.9+80). The fragmentation table shows that the phosphorylated residue corresponds to serine 170 in the PEVK sequence (human N2B cardiac isoform) or S12022 in the full human sequence (Swiss Prot:Q8wz42). (B) Multiple sequence alignments from various species show that Serine170 (red) and its flanking sequence are conserved in all species. Serine 26 (S11878 in the full human sequence) is a conserved serine that was not included in the digestion fragments. (C) In vitro phosphorylation by PKCα kinase of wildtype PEVK, the S170A, S26A and S170/S26A mutants. (Top: Coomassie blue stain gel. Bottom: Autoradiograph). A reduction in 32P incorporation in the mutants can be visibly detected and quantification (D) shows that relative to the wildtype the reduction is highly significant. ***: Significant vs. WT (P<0.001). (n = 9 for wt, S170A, and S170/S26A mutants; n= 5 for S26A).
To further test the involvement of S170 in PEVK phosphorylation, we mutated this site to alanine (S170A). A reduction in 32P incorporation in the S170A mutant following PKCα treatment was clearly detectable (Figure 5C) and gel quantification showed that relative to the wildtype the reduction was ~40% (Figure 5D). This suggests that at least one more PEVK residue in addition to S170 can be phosphorylated by PKCα, but that was not covered by the in-gel-digest-LC/tandem MS experiments (we obtained 53 fragments that covered 46% of the PEVK sequence). Altering the proteolysis conditions, including switching to a different protease (see Supplement), did not increase the coverage. As an alternative approach we performed a sequence analysis of all mammalian species whose titin sequence is currently known and focused on the PEVK region that was not covered by the MS experiments. This revealed a single conserved serine residue, S11878 in full-length human titin, or S26 in the PEVK sequence (see Fig. 5B, left). To evaluate whether it is a PKC substrate we mutated this residue to alanine. The S26A mutant abolished 78% of the wildtype phosphorylation (Fig. 5C) identifying S26 as a PKC substrate. Finally, we also made a S170A/S26A double mutant and found that this virtually abolished phosphorylation (Fig. 5C and 5D).
Discussion
In vitro phosphorylation assays and mass spectrometry both identified the PEVK region as a PKCα substrate, consistent with the finding that PKCα predominately phosphorylates the full length titin molecule and not T2 (largely the A-band region of titin). It is interesting that the constitutively active PKC fragment (PKCcf) did phosphorylate T2, which might be explained by the presence of PKCβ and PKCγ isoforms in the PKCcf protein mixture (in addition to PKCα), or, alternatively, by promiscuity caused by the proteolysis used to generate PKCcf. Although there might be PKC phosphorylation sites elsewhere in the molecule, our work clearly reveals that the PEVK is a prime PKCα phosphorylation site. This is distinct from the PKA site which we have shown to be in the N2B spring element of titin4. This was recently confirmed by Kruger et al., who also showed that PKG phosphorylates the N2B element5. The N2B element is cardiac specific and thus PKA/PKG phosphorylation of titin is therefore restricted to the myocardium. In contrast, the PEVK element identified in our study is constitutively expressed in all isoforms19, including those found in skeletal muscle, and thus its effect is likely to be universal in all striated muscle types. Furthermore, the PKC sites are highly conserved (see also below) unlike the identified PKG site (S469)5 which is only found in human and not in other species. Thus, PKC phosphorylation is expected in all striated muscle types and in a wide range of species.
Mass spectrometry of in-gel-digests of PKCα-phosphorylated PEVK showed that the highly conserved serine S170 is phosphorylated (Fig. 5A), and mutating this site to alanine decreased 32P incorporation in the PEVK element by ~40% (Fig. 5C and D). Because there is still remaining 32P incorporation in the S170A mutant, we also studied S26, which is the only conserved phosphorylation site (either S or T) in the PEVK that was not covered by the in-gel-digest-LC/tandem MS fragments. The S26A mutant decreased 32P incorporation by ~80% suggesting that S26 is an even better PKCα substrate than S170. It is interesting that the two sites, when independently mutated, reduce phosphorylation by more than a combined 100%, which can be explained by a synergistic effect (with mutation in one site lowering phosphorylation of the second site). Overall, our findings establish that S26 and S170 are PKCα substrates that account for the majority of 32P incorporation in the PEVK. The high conservation of S26 as well as S170 (Fig. 5B), supports that these residues perform important biological functions. It is likely that this includes a mechanical function (see below) but other roles such as regulating intermolecular interactions, either between titins, or between titin and other molecules (signaling molecules) can not be excluded and warrants future follow-up work.
PKCα increases passive tension of skinned myocardium. The effect is enhanced by pre-treatment with PP1, which can be explained by the relatively high baseline phosphorylation of titin. Moreover, lowering this baseline by PP1 (Fig. 1D and E) allows for greatly increased PKCα phosphorylation and enhances passive tension. Furthermore, the passive tension effect that results from PKCα phosphorylation is reversible by PP1 treatment. Finally, it is worth noting that PKCα phosphorylation has an effect on passive tension that opposes that of PKA (an increase instead of a decrease). This is analogous to regulatory proteins where, for example, PKA phosphorylation of TnI lowers calcium sensitivity and PKC phosphorylation increases calcium sensitivity20.
The increase in titin-based passive tension following phosphorylation is in agreement with the reduced PL of the single PEVK molecule (PL and force are inversely related, see Methods). Furthermore, the force increase that is predicted from the single molecule experiments (Fig. 4D) has a SL dependence that is similar to that measured in skinned myocardium (Fig. 3B). The mechanism for the PEVK-based force increase is unknown. It has been suggested that the PEVK is an unstructured random coil whose main function is to extend at physiological force levels11, 18, 21. However, a prediction that emanates from this is that sequence drift during evolution is permissible. Instead, the now available titin sequences of a wide region of species indicate that PEVK is highly conserved, especially around S26 and S170 (Fig. 5B). It is therefore possible that the PEVK element is (partially) structured and that this structure is sensitive to PKC phosphorylation. The PL distribution of nonphosphorylated PEVK molecules is fairly broad (Fig 4Ctop), similar to previously reported by Li et al.18, who proposed that this is due to distinct PEVK conformations. Our work supports this view and suggests that PKCα phosphorylation converts conformations with large PL to ones with short PL.
We found that both elastic and viscous stiffness is increased following PKCα phosphorylation, but that elastic stiffness is more responsive (Fig. 3A), suggesting that elasticity and viscosity involve different mechanisms. We previously proposed that titin-based viscosity can be explained by nonspecific electrostatic interactions within the PEVK that can be ruptured in a rate-dependent manner15. The present work suggests that phosphorylation results in an increase in internal PEVK-based crosslinks, which might be due to the negative charges carried by the added phosphate.
In summary, our studies showed that titin is a PKCα substrate, that the PEVK region of titin is a prominent site of PKCα phosphorylation, and that PKCα phosphorylation increases passive tension. Thus, in addition to the known pathways for modulating titin-based stiffness, altering the phosphorylation state of titin's PKC sites constitutes a novel pathway (Fig. 6). The role of this novel PKC pathway for altering passive stiffness under physiological and pathological conditions remains to be established. Clearly it is important to study the phosphorylation state of titin's PEVK in various disease states, including end-stage heart failure, where PKC protein levels and PKC activity are increased9. Inhibiting PKCα has been proposed as a therapeutic strategy for treating heart disease22 and our present findings suggest that improving diastolic function via lowering titin phosphorylation will be one of its benefits.
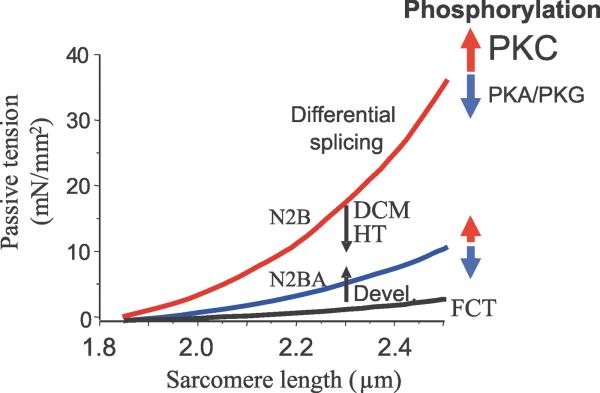
Figure 6. Schematic of titin-based passive stiffness tuning-mechanisms.
Differential splicing gives rise to isoforms of distinct stiffness. During postnatal development (Devel) passive stiffness increases due to switching of fetal cardiac titin (FCT) to adult N2B and N2BA isoforms; hypothyroidism (HT) and dilated cardiomyopathy (DCM) alters splicing in the opposite direction. PKA and PKG phosphorylation reduces passive stiffness and PKC phosphorylation is a novel pathway that increases passive stiffness.
Supplementary Material
Acknowledgements
We thank Joshua Nedrud, Diana Acuna-Wenner, and Luann Wyly for technical help. We especially thank Dr. Chung for the sinusoidal analysis and Dr. Tsaprailis for Mass Spectrometry.
Sources of Funding: NIH grant HL62881(HG), HL77196(MG), and DFG(La668/11-1 to SL). LC-MS/MS data were acquired by the Arizona Proteomics Consortium supported by NIEHS(ES06694), NIH Grant CA23074, and BIO5 Institute of the University of Arizona.
Non-standard Abbreviations and Acronyms
- CL
contour length
- CSA
Cross sectional area
- CTL
control
- δ
phase shift between force and length
- E
complex stiffness and the
- EC50
half maximal effective concentration
- FS
frequency sweep
- kB
Boltzmann's constant,
- LV
left ventricular
- Ig
immunoglobulin
- MHC
myosin heavy chain
- pCa
−log[Ca2+]
- PEVK
proline – glutamate – valine - lysine
- PP1
Protein Phosphatase
- PKC
Protein Kinace C
- PL
persistence length
- SL
sarcomere length
- T
absolute temperature
- WLC
wormlike chain
Footnotes
Disclosures: none.
References
- 1.Solaro RJ. Multiplex kinase signaling modifies cardiac function at the level of sarcomeric proteins. J Biol Chem. 2008;283(40):26829–26833. doi: 10.1074/jbc.R800037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Granzier HL, Labeit S. The giant protein titin: a major player in myocardial mechanics, signaling, and disease. Circ Res. 2004;94(3):284–295. doi: 10.1161/01.RES.0000117769.88862.F8. [DOI] [PubMed] [Google Scholar]
- 3.Wu Y, Cazorla O, Labeit D, Labeit S, Granzier H. Changes in titin and collagen underlie diastolic stiffness diversity of cardiac muscle. J Mol Cell Cardiol. 2000;32(12):2151–2162. doi: 10.1006/jmcc.2000.1281. [DOI] [PubMed] [Google Scholar]
- 4.Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H. Protein kinase A phosphorylates titin's cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res. 2002;90(11):1181–1188. doi: 10.1161/01.res.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]
- 5.Kruger M, Kotter S, Grutzner A, Lang P, Andresen C, Redfield MM, Butt E, Dos Remedios CG, Linke WA. Protein Kinase G Modulates Human Myocardial Passive Stiffness by Phosphorylation of the Titin Springs. Circ Res. 2008 doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda N, Wu Y, Nair P, Granzier HL. Phosphorylation of titin modulates passive stiffness of cardiac muscle in a titin isoform-dependent manner. J Gen Physiol. 2005;125(3):257–271. doi: 10.1085/jgp.200409177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borbely A, Falcao-Pires I, van Heerebeek L, Hamdani N, Edes I, Gavina C, Leite-Moreira AF, Bronzwaer JG, Papp Z, van der Velden J, Stienen GJ, Paulus WJ. Hypophosphorylation of the Stiff N2B Titin Isoform Raises Cardiomyocyte Resting Tension in Failing Human Myocardium. Circ Res. 2009;104(6):780–6. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 8.Murphy S, Frishman WH. Protein kinase C in cardiac disease and as a potential therapeutic target. Cardiol Rev. 2005;13(1):3–12. doi: 10.1097/01.crd.0000124914.59755.8d. [DOI] [PubMed] [Google Scholar]
- 9.Belin RJ, Sumandea MP, Allen EJ, Schoenfelt K, Wang H, Solaro RJ, de Tombe PP. Augmented protein kinase C-alpha-induced myofilament protein phosphorylation contributes to myofilament dysfunction in experimental congestive heart failure. Circ Res. 2007;101(2):195–204. doi: 10.1161/CIRCRESAHA.107.148288. [DOI] [PubMed] [Google Scholar]
- 10.Liu Q, Chen X, Macdonnell SM, Kranias EG, Lorenz JN, Leitges M, Houser SR, Molkentin JD. Protein Kinase C{alpha}, but Not PKC{beta} or PKC{gamma}, Regulates Contractility and Heart Failure Susceptibility. Implications for Ruboxistaurin As a Novel Therapeutic Approach. Circ Res. 2009;105(2):194–200. doi: 10.1161/CIRCRESAHA.109.195313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe K, Nair P, Labeit D, Kellermayer MS, Greaser M, Labeit S, Granzier H. Molecular mechanics of cardiac titin's PEVK and N2B spring elements. J Biol Chem. 2002;277(13):11549–11558. doi: 10.1074/jbc.M200356200. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y, Bogolomovas J, Labeit S, Granzier H. Single molecule force spectroscopy of cardiac titin's N2B element-effects of the molecular chaperone alpha B-crystallin with disease causing mutations. J Biol Chem. 2009 doi: 10.1074/jbc.M809743200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labeit D, Watanabe K, Witt C, Fujita H, Wu Y, Lahmers S, Funck T, Labeit S, Granzier H. Calcium-dependent molecular spring elements in the giant protein titin. Proc Natl Acad Sci U S A. 2003;100(23):13716–13721. doi: 10.1073/pnas.2235652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granzier HL, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J. 1995;68(3):1027–1044. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellermayer MS, Smith SB, Bustamante C, Granzier HL. Mechanical fatigue in repetitively stretched single molecules of titin. Biophys J. 2001;80(2):852–863. doi: 10.1016/S0006-3495(01)76064-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granzier HL, Labeit S. Titin and its associated proteins: the third myofilament system of the sarcomere. Adv Protein Chem. 2005;71:89–119. doi: 10.1016/S0065-3233(04)71003-7. [DOI] [PubMed] [Google Scholar]
- 17.Trombitas K, Freiburg A, Centner T, Labeit S, Granzier H. Molecular dissection of N2B cardiac titin's extensibility. Biophys J. 1999;77(6):3189–3196. doi: 10.1016/S0006-3495(99)77149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Oberhauser AF, Redick SD, Carrion-Vazquez M, Erickson HP, Fernandez JM. Multiple conformations of PEVK proteins detected by single-molecule techniques. Proc Natl Acad Sci U S A. 2001;98(19):10682–10686. doi: 10.1073/pnas.191189098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, Witt CC, Labeit D, Gregorio CC, Granzier H, Labeit S. The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ Res. 2001;89(11):1065–1072. doi: 10.1161/hh2301.100981. [DOI] [PubMed] [Google Scholar]
- 20.Pi Y, Zhang D, Kemnitz KR, Wang H, Walker JW. Protein kinase C and A sites on troponin I regulate myofilament Ca2+ sensitivity and ATPase activity in the mouse myocardium. J Physiol. 2003;552(Pt 3):845–857. doi: 10.1113/jphysiol.2003.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linke WA, Kulke M, Li H, Fujita-Becker S, Neagoe C, Manstein DJ, Gautel M, Fernandez JM. PEVK domain of titin: an entropic spring with actin-binding properties. J Struct Biol. 2002;137(1-2):194–205. doi: 10.1006/jsbi.2002.4468. [DOI] [PubMed] [Google Scholar]
- 22.Hambleton M, York A, Sargent MA, Kaiser RA, Lorenz JN, Robbins J, Molkentin JD. Inducible and myocyte-specific inhibition of PKCalpha enhances cardiac contractility and protects against infarction-induced heart failure. Am J Physiol Heart Circ Physiol. 2007;293(6):H3768–3771. doi: 10.1152/ajpheart.00486.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.