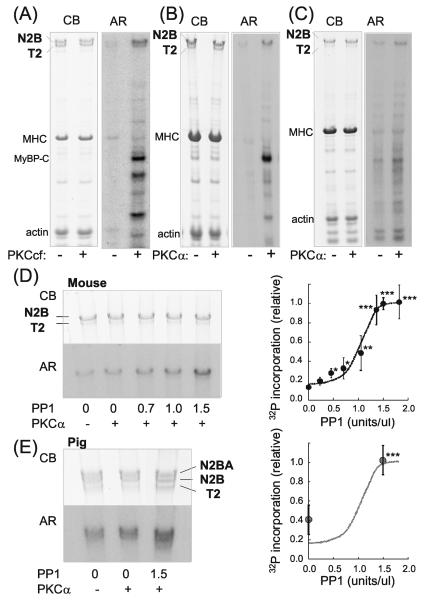
Figure 1. Cardiac titin is phosphorylated by PKCα and dephosphorylated by PP1.
A-C) Representative results on mouse skinned cardiac myocytes (A and B) and skinned myocardium (C) incubated in [γ-32P]ATP without (−) or with (+) PKC. Left panels: Coomassie blue (CB) stained gel and right panels: corresponding autoradiograph (AR). In A) the catalytically active PKC fragment (PKCcf) was used and in B) and C), PKCα. In addition to the well-known PKC substrates MyBP-C and TnI, titin is also phosphorylated. The majority of titin is T1 (upper band representing full-length titin) and a small fraction is T2 (degradation product containing the A-band region of titin). T1 is phosphorylated by both PKCcf and PKCα and T2 only by PKCcf. D and E) Effect of pre-treating skinned myocardium with PP1 on PKCα phosphorylation in mouse (D) and porcine LV (E). 32P incorporation is enhanced by PP1, indicating that PP1 dephosphorylates sites on titin that function subsequently as PKC substrates. Note that pig myocardium coexpresses N2B and N2BA cardiac titin and that both isoforms are phosphorylated by PKCα and dephosphorylated by PP1. Graphs show 32P incorporation relative to the incorporation following pretreatment with 1.5 PP1 U/μl, and normalized by protein loading. The graph in D) reveals that a maximal PP1 effect (n = 5) is seen at ~1.5 U/μl PP1 and E) indicates that at 0 U/μl PP1 pig myocardium (n = 3) is phosphorylated to 40% of maximal, a value several-fold larger than for the mouse (18%). This indicates a higher basal phosphorylation in mouse myocardium than in pig. *significant vs prePKCα. Broken line is the fit to the mouse data of Fig. 1D.