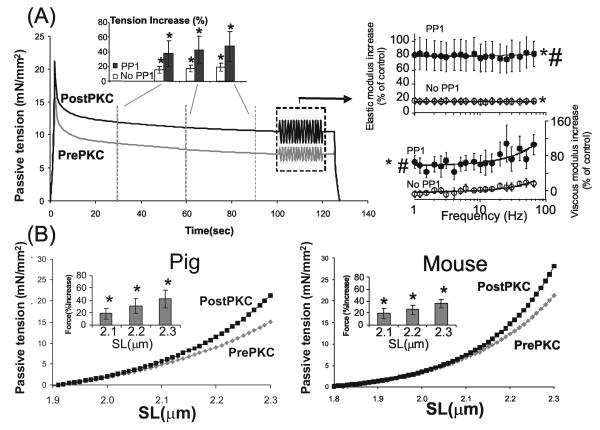
Figure 3. Effect of PKCα phosphorylation on titin-based passive tension in skinned myocardium.
(A) Passive tension during stretch-hold-release protocol before PKCα (prePKC) and after incubation with PKCα (postPKC) in porcine LV skinned myocardium (mean values of 6 fibers). The preparations had been pre-treated with gelsolin to extract thin filaments and prevent actomyosin interaction during and after PKCα treatment. Fibers were preincubated with PP1 to dephosphorylate titin before PKCα incubation. Fibers were stretched from their slack length (SL ~ 1.9 μm) to a SL of 2.3 μm and were then held, followed by a release back to the slack length. Passive tension is significantly higher following PKCα-treatment. Towards the end of the hold phase a frequency sweep was imposed (1-70 Hz, amplitude 1%) (frequency sweep shown is idealized) and elastic and viscous moduli determined. Right: Percent increase in elastic and viscous moduli from pre-PKCα controls, with or without PP1 pre-treatment. Elastic and viscous moduli are higher following PKCα treatment, with PP1 pre-treatment enhancing the effect. (B) Titin-based passive tension-SL relationship measured during stretch of porcine fibers (left panel; mean of 6 muscles) and mouse fibers (right panel; mean of 6 muscles) that had been gelsolin extracted and pre-treated with PP1. The shown mean values indicate that PKCα treatment significantly increases titin-based tension; the increase is small at SLs below ~2.1 μm but progressively increase at longer SLs. Insets show the percent increase in tension at three SLs. * significant vs prePKCα (P<0.05); # Significant vs No PP1.