Abstract
Context
Both higher adherence to a Mediterranean-type diet and more physical activity have been independently associated with lower Alzheimer disease (AD) risk but their combined association has not been investigated.
Objective
To investigate the combined association of diet and physical activity with AD risk.
Design, Setting, and Patients
Prospective cohort study of 2 cohorts comprising 1880 community-dwelling elders without dementia living in New York, New York, with both diet and physical activity information available. Standardized neurological and neuropsychological measures were administered approximately every 1.5 years from 1992 through 2006. Adherence to a Mediterranean-type diet (scale of 0–9; trichotomized into low, middle, or high; and dichotomized into low or high) and physical activity (sum of weekly participation in various physical activities, weighted by the type of physical activity [light, moderate, vigorous]; trichotomized into no physical activity, some, or much; and dichotomized into low or high), separately and combined, were the main predictors in Cox models. Models were adjusted for cohort, age, sex, ethnicity, education, apolipoprotein E genotype, caloric intake, body mass index, smoking status, depression, leisure activities, a comorbidity index, and baseline Clinical Dementia Rating score.
Main Outcome Measure
Time to incident AD.
Results
A total of 282 incident AD cases occurred during a mean (SD) of 5.4 (3.3) years of follow-up. When considered simultaneously, both Mediterranean-type diet adherence (compared with low diet score, hazard ratio [HR] for middle diet score was 0.98 [95% confidence interval {CI}, 0.72–1.33]; the HR for high diet score was 0.60 [95% CI, 0.42–0.87]; P = .008 for trend) and physical activity (compared with no physical activity, the HR for some physical activity was 0.75 [95% CI, 0.54–1.04]; the HR for much physical activity was 0.67 [95% CI, 0.47–0.95]; P = .03 for trend) were associated with lower AD risk. Compared with individuals neither adhering to the diet nor participating in physical activity (low diet score and no physical activity; absolute AD risk of 19%), those both adhering to the diet and participating in physical activity (high diet score and high physical activity) had a lower risk of AD (absolute risk, 12%; HR, 0.65 [95% CI, 0.44–0.96]; P = .03 for trend).
Conclusion
In this study, both higher Mediterranean-type diet adherence and higher physical activity were independently associated with reduced risk for AD.
Previous research has shown that physical activity can slow down or prevent functional decline associated with aging and improve health in older individuals.1,2 However, regarding Alzheimer disease (AD) or dementia, the relationship is less clear, with many studies reporting exercise being associated with lower rates of cognitive decline1,3 or dementia4–7 and others reporting no significant association.8–10 Dietary habits also may play an important role but epidemiological data on diet and AD have been conflicting.11 In this cohort, we previously found that higher adherence to a Mediterranean-type diet is associated with lower risk for AD12,13 and mild cognitive impairment.14
Nevertheless, it is important to know whether physical activity and diet confer independent associations because individuals who exercise often belong to higher educational-socioeconomic strata, are more health conscious, and in general tend to follow healthier eating habits. The magnitude of such potential associations with AD in individuals engaging in such activities is also of great interest from a public health point of view. To our knowledge, there is scarce literature examining diet and exercise combined.
In the current study, we first sought to examine the association between physical activity and risk of AD. We then investigated the extent to which physical activity and adherence to a Mediterranean-type diet had independent associations with AD risk. We hypothesized that both adherence to a Mediterranean-type diet and physical activity would be independently associated with development of AD and that individuals who ate healthfully and participated in physical activity would have additive benefits regarding development of AD.
METHODS
Sample and Diagnoses
The study methods have been described.12,13,15 Briefly, the study included 2 cohorts recruited through the Washington Heights-Inwood Columbia Aging Project (WHICAP) in 1992 and 1999 who were identified (via ethnicity and age stratification processes) from a probability sample of Medicare beneficiaries from 3 contiguous census tracts in northern Manhattan. Recruitment, written informed consent, and study procedures were approved by the institutional review boards of Columbia Presbyterian Medical Center, Columbia University Health Sciences, and the New York State Psychiatric Institute.
At entry, each individual’s medical and neurological history was recorded and a standardized physical and neurological examination was conducted by physicians. All available ancillary information (medical charts, computed tomography scans or magnetic resonance imaging scans) was considered in the evaluation. Additional evaluation instruments included a structured in-person interview including an assessment of health and function and a neuropsychological battery that contained tests of memory (short- and long-term verbal and nonverbal), orientation, abstract reasoning (verbal and nonverbal), language (naming, verbal fluency, comprehension, and repetition), and visual-spatial abilities (copying and matching).16 Using previously described methods,17 data on 15 neuropsychological test variables from the initial visit were grouped into 4 cognitive factors (memory, language, processing speed, and visual-spatial ability), converted into z scores and then averaged to create a composite cognitive z score. A Clinical Dementia Rating (CDR) score also was assigned.
A consensus diagnosis for the presence or absence of dementia was made at a diagnostic conference of neurologists and neuropsychologists based on criteria from the Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised). Standard diagnostic criteria were used for determination of the type of dementia. The diagnosis of probable or possible AD was based on the criteria of the National Institute of Neurological Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association.
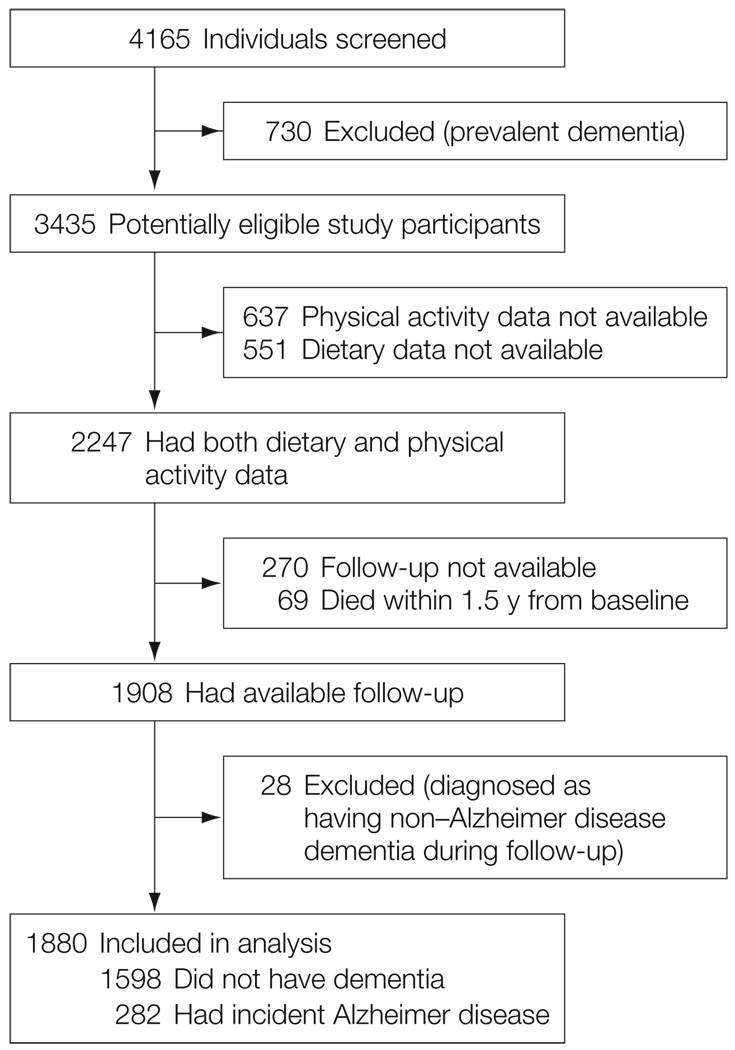
The evaluations were repeated approximately every 1.5 years from 1992 through 2006. Among all potential participants in both WHICAP cohorts, 1880 individuals were considered for this study (FIGURE 1).
Figure 1.
Selection of Individuals for Study Inclusion Who Were From the Washington Heights-Inwood Columbia Aging Project
Physical Activity
Two slightly different versions of the Godin leisure time exercise questionnaire were used.18 Most participants (n=1133) were queried about a 2-week period. The number of times participating and the number of minutes per time participating were recorded for 3 different categories of activities: vigorous (aerobic dancing, jogging, playing handball), moderate (bicycling, swimming, hiking, playing tennis), and light (walking, dancing, calisthenics, golfing, bowling, gardening, horseback riding).
We constructed a summary physical activity score for each individual using the following formula: number of minutes × number of times × coefficient (9 for vigorous, 5 for moderate, and 3 for light activities corresponding to the metabolic equivalent [MET]18). The MET expresses the energy cost consumption during specific physical activities as multiples of resting metabolic rate (obtained during quiet sitting and set by convention to 1 kcal × kg−1 × hr−1 or 1 MET). Physical activities can therefore be classified in terms of intensity based on corresponding METs. In this case, vigorous activities were considered to correspond to 9 METs (or 9 kcal × kg−1 × hr−1), moderate activities to 5 METs (or 5 kcal × kg−1 × hr−1), and light activities to 3 METs (or 3 kcal × kg−1 × hr−1).
Because of skewed distribution, the summary physical activity score was again categorized into tertiles with a similar number of individuals in each group and the following median weekly physical activity values in each group: no physical activity, 0 hours; some physical activity, 0.1 hours of vigorous, 0.8 hours of moderate, or 1.3 hours of light, or a combination thereof; much physical activity, 1.3 hours of vigorous, 2.3 hours of moderate, or 3.8 hours of light, or a combination thereof. Dichotomizing the summary physical activity score resulted in 2 groups with the following median weekly levels: low physical activity, 0 hours; high physical activity, 1.3 hours of vigorous, 2.4 hours of moderate, or 4 hours of light physical activity, or a combination thereof.
A subset of individuals who were recruited earlier in the study (n=747) were queried regarding their physical activity in a different way (number of hours during the most recent month in which they engaged in their typical number of activities). Using procedures similar to the ones described above, a physical activity score was calculated and categorized into tertiles of no physical activity, some physical activity, and much physical activity. Although categorization of individuals in physical activity categories was performed within each version of the questionnaire, in supplementary analyses we additionally explored inclusion of a term representing the period of physical activity assessment in the analyses and considered only the most recent version of the questionnaire.
Test-retest (2 weeks to 1 month) reliability correlation coefficients of the Godin Leisure Time Exercise Questionnaire18 have ranged between 0.62 and 0.81.18–20 Validity of the instrument has been demonstrated in relation to multiple measures including body fat,18,19 maximum oxygen consumption,18,19 Caltrac actometer,19,21 treadmill time,19 and other similar physical activity questionnaires.20,21 Because the questionnaire has not been validated in individuals older than 65 years, we explored its validity in our cohort comparing it with available measures of physical performance: time to complete 5 chair stands (available for 716 individuals), time to walk 1 meter (measured twice and averaged; available for 924 individuals), and time to walk 4 meters (measured twice and averaged; available for 924 individuals). The correlation (Spearman ρ correlation coefficient) of the sum physical activity score was −0.10 with time for chair stands (P = .009), −0.10 with mean time for the 1-meter walk (P = .002), and −0.28 with mean time for the 4-meter walk (P < .001).
Diet
Average food consumption information over the past year was obtained using a 61-item version of the Willett Semiquantitative Food Frequency Questionnaire (Channing Laboratory, Cambridge, Massachusetts).22 We have previously reported validity and reliability of various components of this food frequency questionnaire in WHICAP.23–25
For construction of the Mediterranean-type diet score, we followed the most commonly described method,26 which we also used in our previous work12–14,27 In summary, we first regressed caloric intake (kilocalories) and calculated the derived residuals of daily gram intake for 7 food categories (dairy, meat, fruits, vegetables, legumes, cereals, and fish).26 Individuals were assigned a value of 1 for each beneficial component (fruits, vegetables, legumes, cereals, and fish) whose consumption was at or above the median, for each detrimental component (meat and dairy products) whose consumption was below the median, for a ratio of monounsaturated fats to saturated fats above the median, and for mild to moderate alcohol consumption (>0 to <30 g/d). The diet score was the sum of the scores in the food categories (range, 0–9) with a higher score indicating higher adherence. The diet score was analyzed either in tertiles (low adherence range, 0–3; middle adherence range, 4–5; high adherence range, 6–9) or in a median split (low adherence range, 0–4; high adherence range, 5–9).
Timing of Physical Activity and Diet Score
We used physical activity and diet scores from the first time they were available as the main predictors in the survival analyses. Scores were assessed in the same timeframe overall: the first dietary assessment coincided (approximately every 1.5 years) with the first physical activity assessment for 91% of the individuals; it was performed more than 1.5 years earlier for 7% of the individuals and more than 1.5 years later for 2% of the individuals. Despite this overlap in assessment, because only a single timing variable can be used in survival models, we included as a covariate a term adjusting for the difference in time between first diet and first physical activity assessment. Repeated dietary and physical activity assessments were available for a subset of individuals. These repeated assessments were not used for the main survival analyses but were used in models examining stability of dietary and physical activity reporting over time.
Other Covariates
Age (in years), education (in years), caloric intake (in kilocalories), body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), cohort (1992 cohort was the reference group), sex (male sex was the reference group), apolipoprotein E (APOE) genotype (absence of ε4 allele vs presence of either 1 or 2 ε4 alleles), smoking status at baseline evaluation (no smoking was the reference group), depression (as assessed by physician’s examination; no depression was the reference group), and leisure activities (cognitive and social; continuous score)15 were considered. Ethnic group based on self-report was used as a dummy variable. The categories used were black (non-Hispanic), Hispanic, white (non-Hispanic), or other (white [non-Hispanic] was the reference group). Ethnicity is important for this study because our cohort is multiethnic and there are important educational, genetic, medical, and cultural differences among ethnicities (which may affect dietary and physical activity habits) as well as different dementia rates.28
We calculated a modified version29,30 of the Charlson Index of Comorbidity31 (referred to as comorbidity index), which included items for myocardial infarction, congestive heart failure, peripheral vascular disease, hypertension, chronic obstructive pulmonary disease, arthritis, gastrointestinal tract disease, mild liver disease, diabetes, chronic renal disease, and systemic malignancy from the baseline visit. All items received weights of 1, with the exception of chronic renal disease and systemic malignancy, which received weights of 2. Baseline CDR score (0 vs 0.5) also was considered.
Statistical Analyses
Baseline characteristics of individuals by missing dietary data, dementia status, physical activity, and diet score were compared using the t test or analysis of variance for continuous variables and χ2 test for categorical variables. If the omnibus test was significant in the analysis of variance models examining the association between age, education, caloric intake, BMI, comorbidity index, leisure activities, or diet score (dependent variable) and physical activity tertiles (independent variable), post hoc Bonferroni and Tukey tests were used.
We calculated Cox proportional hazards models with AD as the dichotomous outcome. The time-to-event variable was time from first dietary assessment to first visit with AD diagnosis; persons who did not develop AD were censored at the time of their last follow-up visit. In initial models, physical activity was the main predictor. In subsequent models, both the physical activity and diet scores were considered simultaneously in the same model. In adjusted models, we controlled for the variables of cohort, age, sex, ethnicity, education, BMI, smoking status, depression, leisure activities, comorbidity index, baseline CDR score, APOE, and time between first dietary and first physical activity assessment. Although caloric intake–adjusted residuals were used in the diet score calculation, we also included caloric intake as a covariate in the models. All predictors were used as time-constant covariates. Hence, the term adjusted in this article refers to simultaneous consideration of all of the covariates above.
The Cox models fulfilled the proportionality assumption (Martingale residuals method). The study had 80% power to detect a significant (type I error of .05) either diet or physical activity effect (high vs low) as high as corresponding to a hazard ratio (HR) of 0.78. We then investigated for possible interactions between diet and physical activity on AD risk.
Dichotomous Ratings
According to the 2 × 4 table approach,32 using median cutoffs for physical activity and diet score, individuals were classified into 4 groups as follows: low physical activity plus low diet score; low physical activity plus high diet score; high physical activity plus low diet score; and high physical activity plus high diet score. The variable was entered as a predictor in the Cox models (unadjusted and adjusted) in a dummy form (low physical activity plus low diet score was the reference group). For the trend test calculation, a continuous variable (range, 1–4) was used. An interaction between physical activity and diet score on AD risk was deemed to occur when the observed joint effect differed from that expected.32 The expected joint effect was calculated as the product of the observed independent HRs of physical activity and diet score (multiplicative model) or as the arithmetic sum of the observed independent HRs of physical activity and diet score (additive model).32
Trichotomous Ratings
Using physical activity and diet adherence in their tertile forms, we constructed an additional combined variable classifying individuals as (1) no physical activity plus low diet score; (2) some physical activity plus low diet score or no physical activity plus middle diet score; (3) some physical activity plus middle diet score, or no physical activity plus high diet score, or much physical activity plus low diet score; (4) some physical activity plus high diet score or much physical activity plus middle diet score; and (5) much physical activity plus high diet score. The variable was entered as a predictor in the Cox models in a dummy form using no physical activity plus low diet score as the reference group. For the trend test calculation, a continuous variable (range, 1–5) was used. This study had 80% power to detect a significant (type I error of .05) joint (much physical activity plus high diet score vs other) HR as high as 0.67.
Supplementary Analyses
Adjusted models included a lower number of individuals because of missing data in some of the covariates. To address this issue, we imputed the missing values assuming 2 hypothetical extreme models. In the low-risk model, we assumed that missing values were all in the direction of being associated with low AD risk. In the high-risk model, we assumed that missing values were all in the direction of being associated with high AD risk.
We explored the use of age (rather than duration from baseline assessment) as the timing scale. We performed analyses censoring (rather than excluding) the 28 individuals who developed non-AD dementia. We constructed additional models considering either all dementia diagnoses or considering only AD diagnoses without stroke.
Despite the assignment of individuals in physical activity categories separately within each version of the questionnaire and because of the slight differences in the physical activity questionnaire between the initial and the most recent periods of the study, in exploratory models we included a term representing the period of physical activity assessment (earlier assessment was the reference group). We also repeated the analyses restricting the sample only to individuals with available information from the most recent version of the physical activity questionnaire.
To increase our confidence that dietary and physical activity habits were not affected by early subclinical dementia process (which would make them early manifestations of AD rather than true risk factors), we adjusted for baseline CDR score in all models. In addition, we recomputed the Cox models excluding individuals with mild cognitive deficits (CDR score of 0.5) and less than 2 years of follow-up.
Despite adjusting for many potential confounders, differences in participant characteristics (ie, baseline differences between the different physical activity groups, which are inherent to observational studies due to lack of randomization) may still lead to biased estimates. To further address this, we conducted propensity analyses.33 We computed the propensity scores by using logistic regression with the dependent variable being physical activity level (high vs low) and the independent variables being the covariates used in the adjusted analyses (cohort, age, sex, ethnicity, education, APOE genotype, caloric intake, BMI, smoking status, depression, leisure activities, comorbidity index, and diet score). Propensity scores were categorized into quartiles. Cox proportional hazards models were then used to compare AD incidence rates for categories of physical activity and diet.
These models were stratified for the propensity quartiles and simultaneously adjusted for cohort, age, sex, ethnicity, education, APOE genotype, caloric intake, BMI, smoking status, depression, leisure activities, comorbidity index, baseline CDR score, and time between first dietary and first physical activity assessment. Significance testing for all analyses was 2-sided with a type I error of .05. The statistical software used was SPSS version 16.0 (SPSS Inc, Chicago, Illinois).
RESULTS
Compared with the 1188 individuals who missed either a dietary (n = 551) or physical activity evaluation (n=637), the 2247 individuals with complete dietary and physical activity information (Figure 1) were slightly more educated (9.3 years vs 10.0 years, respectively; P < .001), were more likely to be smokers (7% vs 12%; P < .001), and had better cognitive performance (z score: 0.08 vs 0.24; P < .001), but did not differ statistically in age (76.7 years vs 77.1 years; P = .11), male sex (34% vs 32%; P = .19), ethnicity (25% vs 28% white, 33% vs 32% black, 41 % vs 39% Hispanic, 1% vs 2% other; P = .12), APOE genotype (27% vs 28% ε4 carriers; P = .74), BMI (27.4 vs 27.6; P = .40), comorbidities (1.9 vs 1.9; P = .58), leisure activities (2.1 vs 2.1; P = .82), or depression (6% vs 8%; P = .05).
Compared with the 339 individuals with missing follow-up (69 who died plus 270 who were lost to follow-up; Figure 1), the 1908 individuals with available follow-up were slightly older (76.4 years vs 77.2 years, respectively; P = .05), had lower total caloric intake (1523 vs 1431; P = .008), lower BMI (28.2 vs 27.4; P = .03), fewer comorbidities (2.3 vs 1.9; P < .001), and higher cognitive performance (z score: 0.16 vs 0.25; P = .003), but did not differ statistically by sex (35% male vs 31% male; P = .21), education (9.7 vs 10.1 years; P = .17), ethnicity (28% vs 28% for white, 30% vs 32% for black, 41% vs 38% for Hispanic, 2% vs 2% for other; P = .81), APOE genotype (28% vs 28% ε4 carriers; P = .80), smoking status (12% vs 13%; P = .58), depression (7% vs 8%; P = .65), leisure activities (5.6 vs 5.3; P = .25), diet adherence score (4.4 vs 4.4; P = .80), or physical activity (low: 49% missing vs 47% available; high: 51% missing vs 54% available; P = .40 for low vs high physical activity).
Stability of Physical Activity and Diet Adherence
The main analyses used physical activity and diet scores at the first time they were available. Although we did not use repeated measures of diet12,13 and physical activity in the main analyses, we investigated their changes over time. There were 1015 individuals with multiple dietary assessments who did not develop AD or other dementia during follow-up (2 dietary assessments available for 831 individuals, 3 for 137 individuals, 4 for 43 individuals, and 5 for 4 individuals). The mean (SD) time interval between dietary assessments was 6.1 (3.1) years (range, 1.4–16.4 years). The reported diet score was stable (estimated annual change of the diet score, β = −0.01; P = .44).
There were 155 individuals with multiple dietary assessments who developed dementia during follow-up (2 dietary assessments available for 123 individuals, 3 assessments for 24 individuals, 4 assessments for 6 individuals, and 5 assessments for 2 individuals). The mean (SD) time interval between dietary assessments was 7.8 (3.6) years (range, 1.8–15.8 years) and the reported diet score did not significantly change over time (estimated annual change of the diet score, β = −0.02; P = .34).
Repeated physical activity assessments were available for 923 individuals over a mean (SD) of 3.4 (2.0) years (range, 0.2–11.0 years). Because of the skewed distribution properties of the physical activity measure, we used a nonparametric method to examine changes over time. We used the Wilcoxon signed rank test, which considers information about both the sign and magnitude of the differences between pairs (ie, physical activity reporting at time 1 and physical activity reporting at time 2) and tests the null hypothesis that 2 related medians are the same. Physical activity reporting was largely stable over time (z score: −0.81; P = .12). Expressed differently, physical activity reporting increased (from no physical activity to much physical activity) for 6% and decreased (from much physical activity to no physical activity) for 9%.
Among the 923 individuals with repeated physical activity assessments, 823 did not develop dementia during follow-up, while 100 either had dementia at both physical activity evaluations (n=19) or developed dementia at the second evaluation (n = 81). For the 823 individuals who did not develop dementia, physical activity reporting increased (from no physical activity to much physical activity) for 7% and decreased (from much physical activity to no physical activity) for 8%. For the 100 individuals with dementia, physical activity reporting increased (from no physical activity to much physical activity) for 3% and decreased (from much physical activity to no physical activity) for 15%.
Clinical and Demographic Characteristics
Individuals were followed up for a mean (SD) of 5.4 (3.3) years. Among the 28 individuals with non-AD dementia (Figure 1) who were not included in the analyses, dementia was a result of stroke for 13. A total of 282 individuals developed incident AD. Among them, 39 had coexisting stroke (considered to be a contributor but not the primary cause of dementia).
At first evaluation, compared with individuals who were cognitively normal, those with incident AD were older, less educated, more likely to be Hispanic, less likely to be white, had a lower BMI, and reported slightly more leisure activities (TABLE 1). They also were less physically active. The groups did not differ by sex, APOE genotype, total caloric intake, comorbidities, smoking status, depression, or adherence to a diet. Less physically active individuals were more likely to be female, older, Hispanic, smokers, depressed, and less educated, and had a lower total caloric intake, a higher BMI, and more comorbid illnesses (TABLE 2). Individuals who had lower levels of physical activity adhered less to the diet.
Table 1.
Characteristics of All Individuals at First Evaluation, Stratified by Alzheimer Disease (AD) Incidence
| No. (%) of Individualsa |
P Value |
|||
|---|---|---|---|---|
| No Dementia (n = 1598) |
Incident AD (n = 282) |
All (N = 1880) |
||
| Male sex | 497 (31) | 90 (32) | 587 (31) | .79 |
| Age, mean (SD), y | 76.4 (6.3) | 82 (6.8) | 77.2 (6.6) | <.001 |
| Ethnicity | ||||
| White | 498 (31) | 33 (12) | 531 (28) | <.001 |
| Black | 513 (32) | 92 (33) | 605 (32) | |
| Hispanic | 561 (35) | 154 (55) | 715 (38) | |
| Otherb | 26 (2) | 3 (1) | 29 (2) | |
| Education, mean (SD), y | 10.6 (4.6) | 7.4 (4.4) | 10.1 (4.8) | <.001 |
| ≥1 Apolipoprotein E ε4 allele | 364 (23) | 79 (28) | 443 (24) | .07 |
| Energy, mean (SD), kcal/d | 1424.7 (526.6) | 1465.7 (550.9) | 1430.8 (530.4) | .23 |
| Body mass index, mean (SD)c | 27.6 (5.4) | 26.6 (5.9) | 27.4 (5.5) | .007 |
| Comorbidity index, mean (SD) | 1.1 (1.4) | 1.9 (1.5) | 1.9 (1.4) | .24 |
| Smoker | 199 (13) | 40 (14) | 239 (13) | .42 |
| Depression | 117 (7) | 27 (10) | 144 (8) | .20 |
| Leisure activities, mean (SD) | 5.2 (2.2) | 5.6 (2.1) | 5.3 (2.2) | .005 |
| Mediterranean-type diet score | ||||
| Low (range, 0–3) | 498 (31) | 100 (36) | 598 (32) | .18 |
| Middle (range, 4–5) | 661 (41) | 118 (42) | 779 (41) | |
| High (range, 6–9) | 439 (28) | 64 (23) | 503 (27) | |
| Physical activity | ||||
| No | 418 (26) | 102 (36) | 520 (28) | <.001 |
| Somed | 551 (35) | 99 (35) | 650 (35) | |
| Muche | 629 (39) | 81 (29) | 710 (38) | |
Unless otherwise indicated. Percentages may not equal 100% due to rounding.
Defined as non-white, non-black, American Indian or Pacific Islander, or Asian.
Calculated as weight in kilograms divided by height in meters squared.
Defined as a median of 0.1 hours per week of vigorous, 0.8 hours per week of moderate, or 1.3 hours per week of light physical activity, or a combination thereof.
Defined as a median of 1.3 hours per week of vigorous, 2.4 hours per week of moderate, or 3.8 hours per week of light physical activity, or a combination thereof.
Table 2.
Characteristics of All Individuals at First Evaluation, Stratified by Physical Activity
| Physical Activitya |
P Value |
|||
|---|---|---|---|---|
| No (n = 520) |
Some (n = 650) |
Much (n = 710) |
||
| Male sex | 137 (26) | 188 (29) | 262 (37) | .001 |
| Age, mean (SD), y | 77.9 (6.9)b | 77.6 (6.6)b | 76.3 (6.3)b | <.001 |
| Ethnicity | ||||
| White | 136 (26) | 171 (26) | 224 (32) | .06 |
| Black | 173 (33) | 209 (32) | 223 (31) | |
| Hispanic | 207 (40) | 261 (40) | 247 (35) | |
| Other | 4 (1) | 9 (1) | 16 (2) | |
| ≥1 Apolipoprotein E ε4 allele | 114 (22) | 155 (24) | 174 (25) | .77 |
| Education, mean (SD), y | 9.7 (4.9)b | 9.9 (4.7)b | 10.6 (4.7)b | .001 |
| Energy, mean (SD), kcal/d | 1392.7 (571.1)b | 1389.6 (518.5)b | 1496.5 (503.7)b | <.001 |
| Body mass index, mean (SD)c | 28.3 (6.0)b | 27.5 (5.4)b | 26.7 (5.1)b | <.001 |
| Comorbidity index, mean (SD) | 2.2 (1.5)b | 2.0 (1.4)b | 1.8 (1.4)b | <.001 |
| Smoker | 72 (14) | 71 (11) | 96 (14) | .24 |
| Depression | 58 (11) | 49 (8) | 37 (5) | .001 |
| Leisure activities, mean (SD) | 5.3 (2.1) | 5.4 (2.1) | 5.1 (2.2) | .06 |
| Mediterranean-type diet score | ||||
| Low (range, 0–3) | 190 (37) | 191 (29) | 217 (31) | <.001 |
| Middle (range, 4–5) | 230 (44) | 256 (39) | 293 (41) | |
| High (range, 6–9) | 100 (19) | 203 (31) | 200 (28) | |
Values are expressed as number (percentage) unless otherwise indicated. No physical activity was defined as a median of 0 hours per week. Some physical activity was defined as a median of 0.1 hours per week of vigorous, 0.8 hours per week of moderate, or 1.3 hours per week of light physical activity, or a combination thereof. Much physical activity was defined as a median of 1.3 hours per week of vigorous, 2.4 hours per week of moderate, or 3.8 hours per week of light physical activity, or a combination thereof.
P<.05 for subgroup comparisons indicated with a “b” footnote. These comparisons were calculated by post hoc Bonferroni and Tukey tests. The no and some physical activity groups were compared with the much physical activity group.
Calculated as weight in kilograms divided by height in meters squared.
Physical Activity and Risk for AD
In models considering only physical activity, more physical activity was associated with lower risk for developing AD (TABLE 3). The associations were similar in adjusted and unadjusted models. Compared with physically inactive individuals, report of some physical activity was associated with a 29% to 41% lower risk of developing AD, while report of much physical activity was associated with a 37% to 50% lower risk.
Table 3.
Cox Proportional Hazard Ratios (HRs) for Alzheimer Disease (AD) Incidence by Physical Activity and Mediterranean-Type Diet Scores
| Model | Unadjusted Model |
Adjusted Model |
||||||
|---|---|---|---|---|---|---|---|---|
| No. of Individuals |
HR (95% CI) |
P Value |
No. of Individuals |
HR (95% CI)a |
P Value |
|||
| No Dementia |
Incident AD |
No Dementia |
Incident AD |
|||||
| Physical activityb | ||||||||
| No | 418 | 102 | 1 [Reference] | 308 | 71 | 1 [Reference] | ||
| Some | 551 | 99 | 0.59 (0.45–0.78) | .001 | 445 | 84 | 0.71 (0.51–0.98) | .04 |
| Much | 629 | 81 | 0.50 (0.39–0.67) | <.001 | 499 | 69 | 0.63 (0.45–0.90) | .01 |
| Trend (range, 1 –3) | 1598 | 282 | 0.70 (0.61–0.82) | <.001 | 1252 | 224 | 0.78 (0.67–0.95) | .01 |
| Physical activity + Mediterranean-type diet | ||||||||
| Diet score | ||||||||
| Low (range, 0–3) | 498 | 100 | 1 [Reference] | 397 | 81 | 1 [Reference] | ||
| Middle (range, 4–5) | 661 | 118 | 0.86 (0.66–1.13) | .28 | 508 | 93 | 0.98 (0.72–1.33) | .88 |
| High (range, 6–9) | 439 | 64 | 0.68 (0.50–0.94) | .02 | 347 | 50 | 0.60 (0.42–0.87) | .007 |
| Trend (range, 1–3) | 1598 | 282 | 0.82 (0.71–0.96) | .01 | 1252 | 224 | 0.79 (0.66–0.94) | .008 |
| Physical activityb | ||||||||
| No | 418 | 102 | 1 [Reference] | 308 | 71 | 1 [Reference] | ||
| Some | 551 | 99 | 0.62 (0.47–0.82) | .001 | 445 | 84 | 0.75 (0.54–1.04) | .08 |
| Much | 629 | 81 | 0.52 (0.39–0.70) | <.001 | 499 | 69 | 0.67 (0.47–0.95) | .02 |
| Trend (range, 1–3) | 1598 | 282 | 0.72 (0.62–0.83) | <.001 | 1252 | 224 | 0.82 (0.68–0.97) | .03 |
Abbreviation: CI, confidence interval.
Adjusted models include slightly lower number of individuals because of missing data in some of the covariates. Adjusted models simultaneously control for cohort, age, sex, ethnicity, education, apolipoprotein E ε4 allele, caloric intake, body mass index (calculated as weight in kilograms divided by height in meters squared), smoking, depression, leisure activities, comorbidity index, baseline Clinical Dementia Rating score, and time between first dietary and first physical activity assessment.
No physical activity was defined as a median of 0 hours per week. Some physical activity was defined as a median of 0.1 hours per week of vigorous, 0.8 hours per week of moderate, or 1.3 hours per week of light physical activity, or a combination thereof. Much physical activity was defined as a median of 1.3 hours per week of vigorous, 2.4 hours per week of moderate, or 3.8 hours per week of light physical activity, or a combination thereof.
Physical Activity, Adherence to Diet, and Risk of AD
Both physical activity and diet were significantly associated with AD incidence when considered simultaneously in the same model (either adjusted or unadjusted; Table 3). In these models, belonging to the middle diet adherence tertile was associated with a 2% to 14% risk reduction, while belonging to the highest diet adherence tertile was associated with a 32% to 40% reduced risk. Similarly, compared with individuals with no physical activity, individuals reporting some physical activity had a 25% to 38% lower risk for AD, while individuals reporting much physical activity had a 33% to 48% lower risk for AD.
Interaction Models for Physical Activity and Adherence to Diet
Dichotomous Ratings
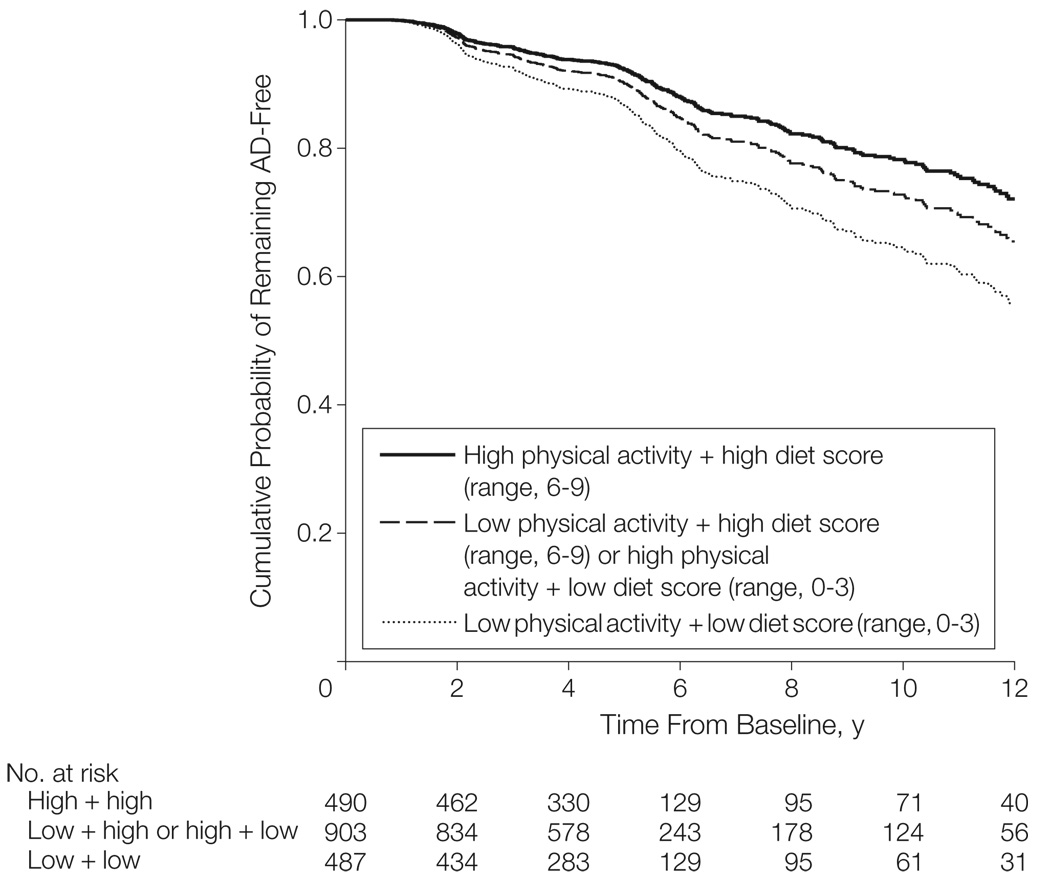
There was a gradual decrease of AD risk with increasing physical activity and diet adherence (TABLE 4 and FIGURE 2). Compared with individuals with low physical activity plus low adherence to diet (absolute AD risk, 19%), high physical activity plus high diet adherence was associated with a 35% to 44% relative risk reduction (absolute AD risk, 12%). The combined physical activity and diet risk reduction was similar to the sum of the individual physical activity and diet associations and did not demonstrate departure from the expected additive effect.32
Table 4.
Cox Proportional Hazard Ratios (HRs) for Alzheimer Disease (AD) Incidence by Physical Activity and Mediterranean-Type Diet Score Dichotomous Ratingsa
| Predictor | Unadjusted Model |
Adjusted Model |
||||||
|---|---|---|---|---|---|---|---|---|
| No. of Individuals |
HR (95% CI) |
P Value |
No. of Individuals |
HR (95% CI)b |
P Value |
|||
| No Dementia |
Incident AD |
No Dementia |
Incident AD |
|||||
| Physical activity + Mediterranean-type dietc | ||||||||
| Low activity + low diet score | 394 | 93 | 1 [Reference] | 301 | 70 | 1 [Reference] | ||
| Low activity + high diet score | 319 | 64 | 0.80 (0.58–1.10) | .17 | 250 | 49 | 0.77 (0.53–1.13) | .18 |
| High activity + low diet score | 452 | 68 | 0.67 (0.49–0.92) | .01 | 363 | 60 | 0.81 (0.57–1.16) | .26 |
| High activity + high diet score | 433 | 57 | 0.56 (0.40–0.78) | .001 | 338 | 45 | 0.65 (0.44–0.96) | .03 |
| Trend (range, 1–4) | 1598 | 282 | 0.85 (0.77–0.95) | .003 | 1252 | 224 | 0.87 (0.77–0.99) | .03 |
|
Adjusted Model |
||||||||
| HR (95% CI)d | HR (95% CI)e | |||||||
| Low activity + low diet score | 266 | 43 | 1 [Reference] | 249 | 41 | 1 [Reference] | ||
| Low activity + high diet score | 217 | 33 | 0.81 (0.51–1.29) | .38 | 202 | 32 | 0.70 (0.50–1.28) | .34 |
| High activity + low diet score | 332 | 38 | 0.68 (0.44–1.07) | .09 | 320 | 33 | 0.61 (0.38–0.97) | .04 |
| High activity + high diet score | 314 | 32 | 0.56 (0.35–0.89) | .02 | 307 | 29 | 0.51 (0.31–0.83) | .006 |
| Trend (range, 1–4) | 1129 | 146 | 0.85 (0.73–0.99) | .04 | 1078 | 135 | 0.83 (0.71–0.98) | .02 |
Abbreviation: CI, confidence interval.
Adjusted models include a slightly lower number of individuals because of missing data in some of the covariates.
Adjusted model simultaneously controls for cohort, age, sex, ethnicity, education, apolipoprotein E ε4 allele, caloric intake, body mass index (calculated as weight in kilograms divided by height in meters squared), smoking, depression, leisure activities, comorbidity index, baseline Clinical Dementia Rating score, and time between first dietary and first physical activity assessment.
Low physical activity was defined as a median of 0 hours per week; high physical activity was defined as a median of 1.3 hours per week of vigorous, 2.4 hours per week of moderate, or 3.8 hours per week of light physical activity, or a combination thereof. The cut point between low and high physical activity is 0.4 hours per week of vigorous, 0.7 hours per week of moderate, or 1.1 hours per week of light physical activity, or a combination thereof. The low Mediterranean-type diet score range is 0 to 4 and the high diet score range is 5 to 9.
Adjusted for everything in footnote “b” except Clinical Dementia Rating score. Individuals with a baseline Clinical Dementia Rating score of 0.5 were excluded from the analyses.
Adjusted for everything in footnote “b” except Clinical Dementia Rating score. Individuals with a baseline Clinical Dementia Rating score of 0.5 and less than 2 years of follow-up were excluded from the analyses.
Figure 2.
Alzheimer Disease (AD) Incidence by High or Low Physical Activity Levels and Mediterranean-Type Diet Adherence Scores
Survival curves are based on Cox analysis. Low physical activity was defined as a median of 0 hours per week of activity; high physical activity, a median of 1.3 hours per week of vigorous, 2.4 hours per week of moderate, or 3.8 hours per week of light activity, or a combination thereof.
Trichotomous Ratings
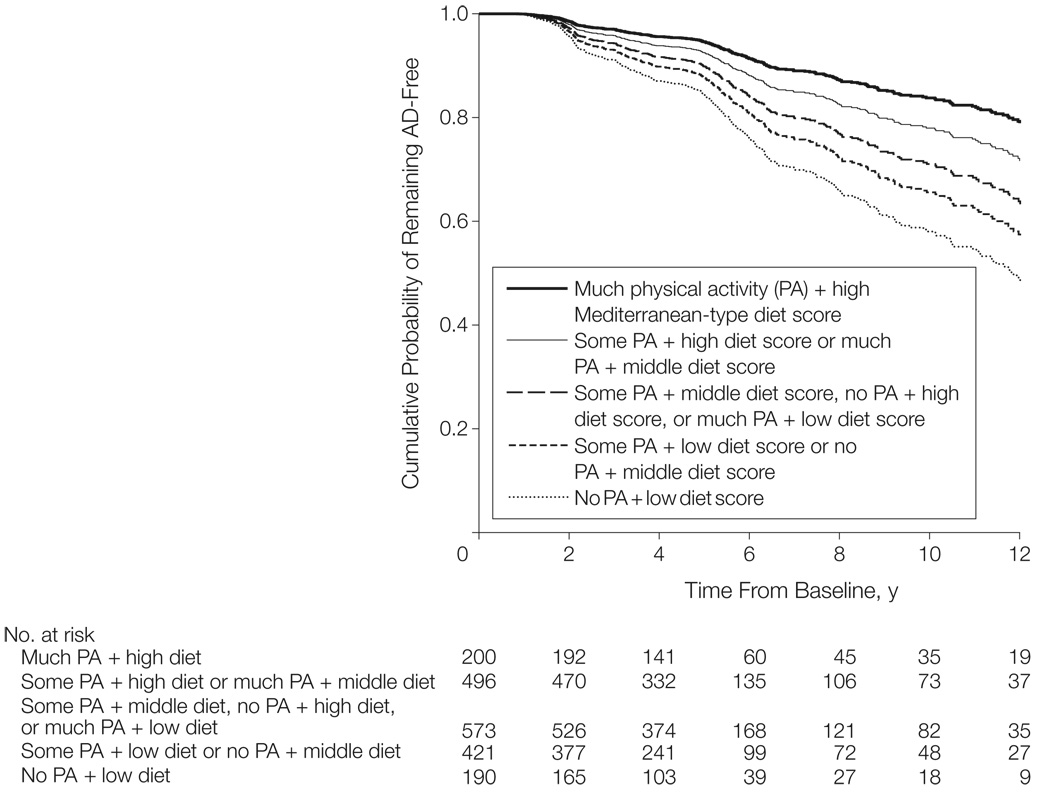
Using no physical activity plus low diet adherence as the reference group, AD risks (expressed as HRs) were 0.78 (95% confidence interval [CI], 0.53–1.14) for some physical activity plus low diet adherence or no physical activity plus middle diet adherence, 0.63 (95% CI, 0.43–0.92) for some physical activity plus middle diet adherence or no physical activity plus high diet adherence or much physical activity plus low diet adherence, 0.46 (95% CI, 0.31–0.69) for some physical activity plus high diet adherence or much physical activity plus middle diet adherence, and 0.33 (95% CI, 0.19–0.57) for much physical activity plus high diet adherence (HR for trend, 0.77 [95% CI, 0.69–0.85]; P < .001 for trend; FIGURE 3).
Figure 3.
Alzheimer Disease (AD) Incidence in Individuals by No, Some, or Much Physical Activity and Low, Middle, and High Mediterranean-Type Diet Adherence Scores
Survival curves are based on Cox analysis. No physical activity was defined as a median of 0 hours per week of activity; some physical activity was defined as median of 0.1 hours per week of vigorous, 0.8 hours per week of moderate, 1.3 hours per week of light activity, or a combination thereof; and much physical activity was defined as a median of 1.3 hours per week of vigorous, 2.4 hours per week moderate, or 3.8 hours per week of light activity, or a combination thereof.
Absolute AD risks declined from 21% in the group with no physical activity plus low diet adherence to 9% in the group with much physical activity plus high diet adherence. The adjusted models produced similar results for AD risks; the HR was 0.96 (95% CI, 0.60–1.55) for some physical activity plus low diet adherence or no physical activity plus middle diet adherence, 0.92 (95% CI, 0.59–1.43) for some physical activity plus middle diet adherence or no physical activity plus high diet adherence or much physical activity plus low diet adherence, 0.58 (95% CI, 0.35–0.95) for some physical activity plus high diet adherence or much physical activity plus middle diet adherence, and 0.39 (95% CI, 0.20–0.76) for much physical activity plus high diet adherence (HR for trend, 0.80 [95% CI, 0.71–0.90]; P < .001 for trend).
Supplementary Analyses
Imputation of missing data in the adjusted analyses did not change the associations. Assuming low risk values of the missing data, compared with individuals with low physical activity plus low diet adherence, those with low physical activity plus high diet adherence had an HR of 0.78 (95% CI, 0.56–1.07), those with high physical activity plus low diet adherence had an HR of 0.74 (0.54–1.03), and those with high physical activity plus high diet adherence had an HR of 0.67 (95% CI, 0.48–0.94) (HR for trend, 0.89 [95% CI, 0.80–0.99]; P = .03 for trend).
Assuming high risk values of the missing data, compared with individuals with low physical activity plus low diet adherence, those with low physical activity plus high diet adherence had an HR of 0.78 (95% CI, 0.56–1.08), those with high physical activity plus low diet adherence had an HR of 0.75 (95% CI, 0.54–1.04), and those with high physical activity plus high diet adherence had an HR of 0.66 (95% CI, 0.47–0.94) (HR for trend, 0.88 [95% CI, 0.79–0.99]; P = .03 for trend).
Using age as the time scale in adjusted models produced the following results. Compared with individuals with low physical activity plus low diet adherence, those with low physical activity plus high diet adherence had an HR of 0.74 (95% CI, 0.50–1.08), those with high physical activity plus low diet adherence had an HR of 0.95 (95% CI, 0.67–1.37), and those with high physical activity plus high diet adherence had an HR of 0.71 (95% CI, 0.48–1.04) (HR for trend, 0.88 [95% CI, 0.78–0.99]; P = .04 for trend). The association of diet and physical activity with AD incidence was attenuated, underlining the prominent effect of age in this neurodegenerative disease, but it persisted as a trend, suggesting an additional residual influence of diet and physical activity.
Considering the 28 cases of non-AD dementia as censored (rather than excluding them; Figure 1) produced the following results. In fully adjusted models, compared with individuals with low physical activity plus low diet adherence, those with low physical activity plus high diet adherence had an HR of 0.80 (95% CI, 0.55–1.17), those with high physical activity plus low diet adherence had an HR of 0.83 (95% CI, 0.55–1.17), and those with high physical activity plus high diet adherence had an HR of 0.65 (95% CI, 0.44–0.96) (HR for trend, 0.88 [95% CI, 0.78–0.99]; P = .03 for trend).
Repeating the analyses including all individuals with dementia (ie, 28 + 282 = 310; Figure 1) did not change the results. In fully adjusted models, compared with individuals with low physical activity plus low diet adherence, those with low physical activity plus high diet adherence had an HR of 0.76 (95% CI, 0.53–1.08), those with high physical activity plus low diet adherence had an HR of 0.81 (95% CI, 0.57–1.13), and those with high physical activity plus high diet adherence had an HR of 0.62 (95% CI, 0.43–0.90) (HR for trend, 0.86 [95% CI, 0.77–0.97]; P = .01 for trend).
Repeating the analyses considering only AD cases without stroke (ie, 282−39 = 243) also did not change the results. In fully adjusted models, compared with individuals with both low physical activity plus low diet adherence, those with low physical activity plus high diet adherence had an HR of 0.67 (95% CI, 0.45–1.00), those with high physical activity plus low diet adherence had an HR of 0.75 (95% CI, 0.51–1.11), and those with high physical activity plus high diet adherence had an HR of 0.65 (95% CI, 0.43–0.98) (HR for trend, 0.86 [95% CI, 0.76–0.99]; P = .03 for trend).
Adding a term for the period of physical activity assessment in adjusted models produced the following results. Compared with individuals with low physical activity plus low diet adherence, those with low physical activity plus high diet adherence had an HR of 0.78 (95% CI, 0.54–1.14), those with high physical activity plus low diet adherence had an HR of 0.82 (95% CI, 0.58–1.18), and those with high physical activity plus high diet adherence had an HR of 0.67 (95% CI, 0.46–0.99) (HR for trend, 0.88 [95% CI, 0.78–0.99] P = .04 for trend).
Restricting the analyses only to individuals with the most recent version of the physical activity questionnaire made the associations even stronger. In adjusted models, compared with individuals with low physical activity plus low diet adherence, those with low physical activity plus high diet adherence had an HR of 0.60 (95% CI, 0.39–0.95), those with high physical activity plus low diet adherence had an HR of 0.55 (95% CI, 0.33–0.92), and those with high physical activity plus high diet adherence had an HR of 0.51 (95% CI, 0.30–0.87) (HR for trend, 0.81 [95% CI, 0.69–0.96]; P = .01 for trend).
The associations remained similar in models excluding individuals with baseline evidence of mild cognitive deficits (CDR score of 0.5; Table 4). When both persons with a CDR score of 0.5 and those followed up for less than 2 years were excluded, despite lower power, the results were similar (Table 4).
Propensity analyses that stratified for high and low physical activity groups matched on demographic and clinical characteristics and adjusted for all co-variates produced similar results. Compared with individuals with low physical activity plus low diet adherence, those with low physical activity plus high diet adherence had an HR of 0.78 (95% CI, 0.53–1.14), those with high physical activity plus low diet adherence had an HR of 0.86 (95% CI, 0.60–1.23), and those with high physical activity plus high diet adherence had an HR of 0.66 (95% CI, 0.44–0.98) (HR for trend, 0.87 [95% CI, 0.77–0.99]; P = .04 for trend).
COMMENT
This study suggests that more physical activity is associated with a reduction in risk for developing AD. The gradual reduction in risks for higher tertiles of physical activity also suggests a possible dose-response association. Elderly individuals are often quite physically inactive. High physical activity in this cohort of 77-year-old individuals corresponded to approximately 1.3 hours of vigorous physical activity per week, 2.4 hours of moderate physical activity per week, or 4 hours of light physical activity per week, or a combination thereof. Nevertheless, even this relatively small amount of physical activity was associated with a reduction in risk for developing AD.
Although some studies have failed to detect an association between physical activity and cognition,8–10 our results are in accordance with many other studies in the literature that suggest a potentially beneficial role for physical activity regarding either rates of cognitive decline1,3 or dementia.4–7 Cognitive benefits for physical activity have been demonstrated even in small preliminary intervention studies either in healthy elders34 or in those with cognitive impairment or dementia.35,36 Nevertheless, clinical trial evidence for a protective effect of physical activity is still insufficient overall.37
Evidence for connections between physical activity and brain biology is abundant. Cardiovascular fitness has been related to lower age-related brain atrophy in structural magnetic resonance imaging,38 to differential patterns of activation suggesting improved plasticity in functional magnetic resonance imaging,39 to increased cerebral blood flow,40 and to increased cerebral blood volume in the dentate gyrus (suggesting possibly increased neurogenesis).41 Animal studies have suggested that exercise may promote angiogenesis,42 neurogenesis, synaptic plasticity and learning,41 neuronal survival and resistance to brain insults,43 and may increase levels of brain-derived neurotrophic factor and expression of genes that could benefit plasticity.44,45 Higher physical activity also has been associated with reduction of inflammation,46 increased concentration of various neurotransmitters,47,48 and increased insulin growth factor.49 Physical activity has even been associated with AD pathological changes in mice; exercise has been shown to result in decreased cortical amyloid burden, possibly mediated by a change in the processing of amyloid precursor protein.50
Physical activity is only one of the factors constituting a healthy lifestyle. Another important one is dietary habits. More health-conscious individuals often follow not one but many aspects of healthy behavior. Therefore, the investigation of which particular dimensions of lifestyle are associated with disease risk is important. Nevertheless, many studies focus on specific individual factors. One of the reasons for the failure to consider diet in the past is the difficulty in summarizing dietary habits. This is particularly true in the neurological and dementia literature in which the methodological tool of dietary patterns has been underused. An exception to this is the Mediterranean-type diet pattern, which we previously reported to be associated with lower risk for AD,12,13 mild cognitive impairment,14 and lower mortality in AD.27
Dietary patterns reflect better everyday dietary habits (ie, foods or nutrients are not consumed in isolation but rather as components of an overall diet), and capture the diet’s multidimensionality because they can integrate complex or subtle interactive effects of many dietary constituents and bypass problems generated by multiple testing and the high correlations that may exist among these constituents. In addition to this, as demonstrated in the present study, individuals’ dietary habits can be effectively summarized in single scores that can be examined in terms of disease risk in the face of other potential predictors.
Because participating in physical activity and healthy eating are often related to each other (as shown in the present study), it could be argued that the association between physical activity and AD is just a manifestation of more physically active individuals eating healthier. Nevertheless, their association with lower rates of AD development was independent of each other. The highest tertiles for both physical activity and Mediterranean-type diet were associated with a 61% to 67% lower risk of AD, an association present after adjusting for multiple potential confounders. Therefore, it seems that both eating well and participating in physical activity may independently confer AD-related health benefits.
Study limitations relating to the construction of the Mediterranean-type diet score (use of an a priori dietary pattern score, equal weighting of underlying food categories, underestimating total food and caloric intake, etc) have been discussed.12,13 Physical activity was more weighted toward leisure-recreational type of activities, while the contribution of physical components of everyday activities was not recorded. Physical activity was based on reporting and not on physiological measurement of maximum oxygen consumption or other objective methods. Nevertheless, it correlated with objective measures of physical performance. To the extent that physical activity measurement error is unrelated to AD outcome, this may bias our results toward the null. Two different variants of the physical activity assessment instrument were used, but the associations remained in models adjusted for this or when we used only one of the physical activity variants.
Follow-up was relatively short and reverse causality or recall bias from persons with early subclinical cognitive deficits cannot be excluded. Both the dietary and physical activity measures demonstrated relative stability over time, but individuals who developed dementia reported somewhat higher decline in both physical activity and Mediterranean-type diet adherence. Nevertheless, adjusting for baseline CDR score and sensitivity analyses excluding individuals with mild cognitive impairment and/or considering a 2-year lag did not attenuate the associations.
Individuals not included in the present analyses because of either missing physical activity and dietary information or lack of follow-up did not differ in many characteristics but were slightly less educated, less likely to be smokers, younger, and had lower cognitive performance, higher caloric intake, higher BMI, and more comorbidities. The above characteristics have variable bidirectional associations with the outcome because low cognitive performance, low education,15 and higher caloric intake23 are risk factors for AD, while younger age and higher BMI are protective. Additionally, potential confounding was addressed by adjusting for all the above factors. Nevertheless, selection bias due to healthier individuals remaining in the cohort is possible. All observational epidemiology studies have residual confounding, in particular of the healthy person type, which cannot be excluded. This issue can only be definitively addressed by randomized controlled trials.
Confidence in our findings is strengthened by the following factors. The study is community-based and the population is multiethnic, increasing the external validity of the findings. Assessment instruments that have been previously validated and widely used in epidemiological studies were applied. The diagnosis of AD took place in a university hospital with expertise in dementia and was based on comprehensive assessments and standard research criteria. The patients were followed up prospectively at relatively short intervals. Measures for multiple potential confounders were carefully recorded and adjusted for in the analyses. Using a variety of sensitivity analyses, including conservative propensity analyses methods, the results were similar.
In summary, our results support the potentially independent and important role of both physical activity and dietary habits in relation to AD risk. These findings should be further evaluated in other populations.
Acknowledgments
Funding/Support: The study was supported by National Institute on Aging grants AG028506 and P01-AG07232.
Role of the Sponsor: The National Institute on Aging financially supported the design and conduct of the study, the collection, management, analysis, and interpretation of the data, and the preparation of the manuscript, but was not involved in manuscript review or approval.
Footnotes
Author Contributions: Dr Scarmeas had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Scarmeas, Luchsinger, Brickman, Stern.
Acquisition of data: Scarmeas, Schupf, Stern.
Analysis and interpretation of data: Scarmeas, Luchsinger, Schupf, Brickman, Cosentino, Tang, Stern.
Drafting of the manuscript: Scarmeas, Brickman.
Critical revision of the manuscript for important intellectual content: Luchsinger, Schupf, Cosentino, Tang, Stern.
Statistical analysis: Scarmeas, Schupf, Cosentino, Tang.
Obtained funding: Scarmeas, Luchsinger, Stern.
Administrative, technical, or material support: Stern.
Study supervision: Scarmeas.
Reprints/E-prints
reprints@ama-assn.org
Financial Disclosures: None reported.
REFERENCES
- 1.Middleton LE, Mitnitski A, Fallah N, et al. Changes in cognition and mortality in relation to exercise in late life. PLoS One. 2008;3(9):e3124. doi: 10.1371/journal.pone.0003124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hakim AA, Petrovitch H, Burchfiel CM, et al. Effects of walking on mortality among nonsmoking retired men. N Engl J Med. 1998;338(2):94–99. doi: 10.1056/NEJM199801083380204. [DOI] [PubMed] [Google Scholar]
- 3.Weuve J, Kang JH, Manson JE, et al. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292(12):1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 4.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144(2):73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 5.Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk. Am J Epidemiol. 2005;161(7):639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 6.Rovio S, Kareholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Larson EB, Bowen JD, van Belle G. Performance-based physical function and future dementia in older people. Arch Intern Med. 2006;166(10):1115–1120. doi: 10.1001/archinte.166.10.1115. [DOI] [PubMed] [Google Scholar]
- 8.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 9.Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia. Am J Epidemiol. 2002;155(12):1081–1087. doi: 10.1093/aje/155.12.1081. [DOI] [PubMed] [Google Scholar]
- 10.Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287(6):742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 11.Luchsinger JA, Noble JM, Scarmeas N. Diet and Alzheimer’s disease. Curr Neurol Neurosci Rep. 2007;7(5):366–372. doi: 10.1007/s11910-007-0057-8. [DOI] [PubMed] [Google Scholar]
- 12.Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol. 2006;63(12):1709–1717. doi: 10.1001/archneur.63.12.noc60109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scarmeas N, Stern Y, Tang MX, et al. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006;59(6):912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scarmeas N, Stern Y, Mayeux R, et al. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66(2):216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scarmeas N, Levy G, Tang MX, et al. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001;57(12):2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stern Y, Andrews H, Pittman J, et al. Diagnosis of dementia in a heterogeneous population. Arch Neurol. 1992;49(5):453–460. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- 17.Scarmeas N, Albert SM, Manly JJ, Stern Y. Education and rates of cognitive decline in incident Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006;77(3):308–316. doi: 10.1136/jnnp.2005.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10(3):141–146. [PubMed] [Google Scholar]
- 19.Jacobs DR, Jr, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25(1):81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Sallis JF, Buono MJ, Roby JJ, et al. Seven-day recall and other physical activity self-reports in children and adolescents. Med Sci Sports Exerc. 1993;25(1):99–108. doi: 10.1249/00005768-199301000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Miller DJ, Freedson PS, Kline GM. Comparison of activity levels using the Caltrac accelerometer and five questionnaires. Med Sci Sports Exerc. 1994;26(3):376–382. [PubMed] [Google Scholar]
- 22.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 23.Luchsinger JA, Tang MX, Shea S, Mayeux R. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59(8):1258–1263. doi: 10.1001/archneur.59.8.1258. [DOI] [PubMed] [Google Scholar]
- 24.Luchsinger JA, Tang MX, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch Neurol. 2003;60(2):203–208. doi: 10.1001/archneur.60.2.203. [DOI] [PubMed] [Google Scholar]
- 25.Luchsinger JA, Tang MX, Siddiqui M, et al. Alcohol intake and risk of dementia. J Am Geriatr Soc. 2004;52(4):540–546. doi: 10.1111/j.1532-5415.2004.52159.x. [DOI] [PubMed] [Google Scholar]
- 26.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348(26):2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 27.Scarmeas N, Luchsinger JA, Mayeux R, Stern Y. Mediterranean diet and Alzheimer disease mortality. Neurology. 2007;69(11):1084–1093. doi: 10.1212/01.wnl.0000277320.50685.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang MX, Stern Y, Marder K, et al. The APOE-ε4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279(10):751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- 29.Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62(10):1601–1608. doi: 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scarmeas N, Albert M, Brandt J, et al. Motor signs predict poor outcomes in Alzheimer disease. Neurology. 2005;64(10):1696–1703. doi: 10.1212/01.WNL.0000162054.15428.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 32.Botto LD, Khoury MJ. Commentary: facing the challenge of gene-environment interaction. Am J Epidemiol. 2001;153(10):1016–1020. doi: 10.1093/aje/153.10.1016. [DOI] [PubMed] [Google Scholar]
- 33.D’Agostino RB, Jr, D’Agostino RB., Sr Estimating treatment effects using observational data. JAMA. 2007;297(3):314–316. doi: 10.1001/jama.297.3.314. [DOI] [PubMed] [Google Scholar]
- 34.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults. Psychol Sci. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 35.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia. Arch Phys Med Rehabil. 2004;85(10):1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 36.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease. JAMA. 2008;300(9):1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 37.Forbes D, Forbes S, Morgan DG, Markle-Reid M, Wood J, Culum I. Physical activity programs for persons with dementia. Cochrane Database Syst Rev. 2008;(3):CD006489. doi: 10.1002/14651858.CD006489.pub2. [DOI] [PubMed] [Google Scholar]
- 38.Colcombe SJ, Erickson KI, Raz N, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58(2):176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 39.Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101(9):3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rogers RL, Meyer JS, Mortel KF. After reaching retirement age physical activity sustains cerebral perfusion and cognition. J Am Geriatr Soc. 1990;38(2):123–128. doi: 10.1111/j.1532-5415.1990.tb03472.x. [DOI] [PubMed] [Google Scholar]
- 41.Pereira AC, Huddleston DE, Brickman AM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104(13):5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Black JE, Isaacs KR, Anderson BJ, et al. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A. 1990;87(14):5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. 2001;21(15):5678–5684. doi: 10.1523/JNEUROSCI.21-15-05678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726(1–2):49–56. [PubMed] [Google Scholar]
- 45.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 46.Reuben DB, Judd-Hamilton L, Harris TB, et al. The associations between physical activity and inflammatory markers in high-functioning older persons. J Am Geriatr Soc. 2003;51(8):1125–1130. doi: 10.1046/j.1532-5415.2003.51380.x. [DOI] [PubMed] [Google Scholar]
- 47.Blomstrand E, Perrett D, Parry-Billings M, Newsholme EA. Effect of sustained exercise on plasma amino acid concentrations and on 5-hydroxytryptamine metabolism in six different brain regions in the rat. Acta Physiol Scand. 1989;136(3):473–481. doi: 10.1111/j.1748-1716.1989.tb08689.x. [DOI] [PubMed] [Google Scholar]
- 48.Fordyce DE, Farrar RP. Physical activity effects on hippocampal and parietal cortical cholinergic function and spatial learning in F344 rats. Behav Brain Res. 1991;43(2):115–123. doi: 10.1016/s0166-4328(05)80061-0. [DOI] [PubMed] [Google Scholar]
- 49.Borst SE, Vincent KR, Lowenthal DT, Braith RW. Effects of resistance training on insulin-like growth factor and its binding proteins in men and women aged 60 to 85. J Am Geriatr Soc. 2002;50(5):884–888. doi: 10.1046/j.1532-5415.2002.50215.x. [DOI] [PubMed] [Google Scholar]
- 50.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25(17):4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]