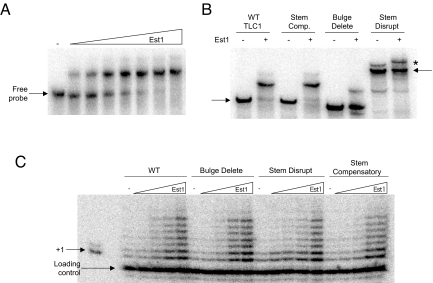
Fig. 2.
Est1 RNA binding is dependent upon the central TLC1 bulged-stem loop. (A) To assess the Est1 RNA binding activity EMSA was used in conjunction with a radiolabeled TLC1 RNA probe. To measure the interaction affinity between Est1 and TLC1 the wild-type bulged-stem loop RNA (250 pM) was incubated alone or with varying Est1 concentrations (30, 60, 120, 240, 480, 960, and 1,440 nM). All binding reactions were resolved by native polyacrylamide electrophoresis. The arrows mark free probe locations. (B) The Est1 RNA binding specificity was determined using established TLC1 RNAs that included stem disruption, bulge deletion and stem compensatory mutants (13). The slower migration for the stem-disruption RNA indicates that it is in a distinct conformation. The ability of Est1 (480 nM) to bind to each TLC1 derivative (250 pM) was determined by RNA EMSA. The asterisk marks the position of a minor slower migrating, distinctly folded RNA that occurs with the stem disrupt RNA. Although the migration of minor species reproducibly slows further following incubation with Est1, the change in migration is not dependent upon a stable Est1 interaction as it is apparent even after degradation of the Est1 following protease addition. Hence, the slight migration shift in the minor RNA species present in the stem disrupt experiment does not result from a stable association with Est1. (C) The Est1 effect on telomerase enzymes with the full-length TLC1 bulged-stem loop derivatives was tested using in vitro DNA extension assays and a 7-base 3′-overhang DNA substrate. Reactions were supplemented with increasing Est1 amounts (1, 10, 100, or 200 nM), as designated.