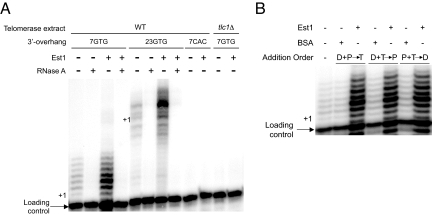
Fig. 3.
Est1 upregulates the telomerase DNA extension activity independent of DNA interactions. (A) The capacity of Est1 (50 nM) to stimulate telomerase-mediated DNA extension using substrates with either 7- or 23-base single-stranded 3′-overhang lengths was determined. To demonstrate that the DNA extension products were telomerase-dependent the telomerase extracts were pretreated with RNaseA, a DNA substrate with a C-rich 3′-overhang was incorporated or extract prepared from tlc1Δ yeast was used in the extension reactions, as indicated. (B) The Est1 ability to affect free or DNA-bound telomerase was determined by altering the order of addition for Est1/BSA protein (P) (50 nM), 7-base DNA substrate (D) and telomerase extract (T). The two components that were preincubated (2 min r.t.) are group followed by the factor that was added with free nucleotide to initiate the reaction. The positions of the loading control primer and the various + 1 DNA extension products are marked.