Fig. 4.
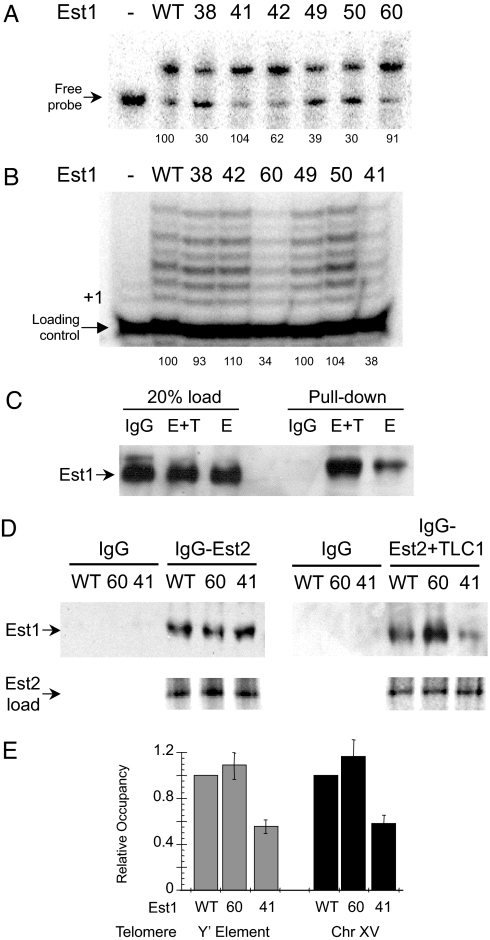
Defects in nucleic acid binding and telomerase stimulation are non-correlative for Est1 derivatives. The Est1 point mutants Est1–38, Est1–41, Est1–42, Est1–49, Est1–50, and Est1–60 were purified to near homogeneity and characterized. (A) The RNA binding activity of each derivative (100 nM) was determined by EMSA using a radiolabeled TLC1 RNA (250 pM). (B) The ability of the various Est1 proteins (50 nM) to enhance telomerase DNA extension activity was examined. The positions of + 1 and the loading control primer are specified. (C) The capacity of Est1 to interact with Est2 independent of Tlc1 RNA was examined in vitro. Est1 (500 ng) was added to either IgG Sepharose alone or IgG Sepharose preincubated with either ProA-Est2 + Tlc1 (E+T) or ProA-Est2 (E). Est1 loading (20% input) control data are shown. (D) The abilities of wild-type Est1, Est1–60 and Est1–41 to interact with Est2 in the absence or presence of TLC1 RNA were determined using Est2 protein immobilized on IgG Sepharose. Representative data from three independent experiments is shown. For the pull-down experiments the Est1 proteins were visualized by Western blot analysis using an antibody directed against the His6-tag. Est2 loading control data are shown where the [35S]Met-labeled Est2 translation products were resolved by SDS/PAGE and visualized using a PhosphoImager. (E) Telomere association by the Est1 derivatives was monitored using the ChIP assay. Est1 residencies at a telomere population were determined using PCR and primers select for a subtelomeric Y' element found at 11 chromosomal termini (gray bars) or at a single telomere using primers specific for a subtelomeric region of chromosome XV (black bars); values were normalized to the signal from an internal non-telomeric DNA. All data represent average values (mean ± SD) from three independent assays.