Abstract
Cigarette smoking and caffeine use are established and problematic drug-use behaviors in people with schizophrenia. Associative links between drugs of abuse may occur but the relationship between caffeine use and cigarette smoking has received little attention in schizophrenia. In this cross-cue reactivity laboratory study, we examined the effects of neutral and smoking cues on craving for caffeinated beverages in participants with schizophrenia or schizoaffective disorder (SS; n = 15) and non-psychiatric controls (CS; n = 18) all of whom were heavy smokers and daily caffeine users. Participants were tested under non-abstinent and 5-hour abstinent conditions. SS tended to report greater daily levels of caffeine use than CS. Although this difference was not significant, that may be due to the small sample sizes as the size of this effect was large. Daily caffeine intake was significantly correlated with daily smoking rate in SS but not CS. A significant interaction between group and cue type after controlling for caffeine intake indicated that exposure to smoking cues increased urge for caffeinated beverages in SS but not CS. These results indicate support for associative connections between cigarette smoking cues and craving for caffeine in smokers with schizophrenia.
Keywords: caffeine, nicotine, smoking, tobacco use disorder, schizophrenia, cue reactivity
1. Introduction
In people with schizophrenia, substance abuse is an established and problematic behavior that is related to poorer overall mental and physical health functioning (Winklbaur et al., 2006). For example, recent attention has pointed to increased rates of cigarette smoking (de Leon and Diaz, 2005; Kotov et al., 2008), alcohol (Eriksson et al., 2007), cocaine (Shaner et al., 1995) and marijuana (Degenhardt and Hall, 2002) use in this population. Elevated caffeine use has also been reported (e.g., Benson et al., 1986; Zaslove et al., 1991). Cross-sectional studies have found that people with schizophrenia are more likely to be daily consumers of caffeinated beverages, or are more likely to consume higher caffeine doses, than those without psychiatric illness (Gurpegui et al., 2006; Hughes et al., 1998; Rihs et al., 1996; Schneier and Siris, 1987; Strassnig et al., 2006). High levels of caffeine use can be problematic for people with schizophrenia as caffeine can increase psychiatric symptoms, antipsychotic plasma levels, and medication side effects (de Leon 2004; Hughes et al., 1998).
People with schizophrenia may consume high levels of caffeine for a number of reasons. Caffeine indirectly increases dopamine (DA) neurotransmission (Tanda and Goldberg, 2000), and functional hyperactivity of the mesolimbic DA system associated with schizophrenia may predispose these patients toward experiencing stronger reinforcing effects of DA-releasing drugs and drug-related cues (Chambers et al., 2001). People with schizophrenia also may use caffeine for its beneficial effects on alertness and cognition (Haskell et al., 2005). Furthermore, schizophrenia is associated with polydipsia, which may non-specifically increase caffeine consumption (de Leon et al., 1994; 2002).
Another likely contributor is the high rate of cigarette smoking in this population, as caffeine and cigarette consumption are strongly associated in smokers with schizophrenia and in non-psychiatric smokers (Gurpegui et al., 2006; Istvan and Matarazzo, 1984; Strassnig et al., 2006). Several mechanisms may underlie this association. Although the genetic correlation between use of these substances appears to be modest (Kendler et al., 2008), impulsivity is associated with heavy use of both substances (Gurpegui et al., 2007). Smoking induces the cytochrome P450 isoenzyme CYP1A2, which metabolizes caffeine, so that smokers require more caffeine than non-smokers to obtain the desired effects of caffeine (de Leon et al., 2003, 2004). Furthermore, caffeine enhances the stimulating and reinforcing effects of nicotine in preclinical animal models (Shoaib et al., 1999; Tanda and Goldberg, 2000) and controlled human laboratory studies (Duka et al., 1998; Jones and Griffiths, 2003; Perkins et al., 1994), albeit not consistently (e.g., Chait and Griffiths, 1983; Perkins et al., 2005).
Naturalistic studies indicate that caffeine and nicotine are often used at the same time (Emurian et al., 1982; Nellis et al., 1982; Marshall et al., 1980). Through associative learning during their concurrent consumption, smoking-associated stimuli should come to trigger urges for caffeine use and vice versa, just as smoking cues increase craving for alcohol and vice versa in people who use those substances (e.g., Colby et al., 2004; Drobes, 2002). In this study, we examined the effects of smoking cues on urge to consume caffeinated beverages in smokers with schizophrenia (SS) and equally-heavy control smokers without psychiatric illness (CS), all of whom were daily caffeine users. We hypothesized that smoking cues would increase caffeine urges due to associative learning processes arising from concurrent self-administration of these substances. Based on the theory that schizophrenia is associated with increased responsivity to drugs and drug-related cues (Chambers et al., 2001), we further hypothesized that SS would have higher caffeine urges and would be more prone to experiencing effects of smoking cues on caffeine urges than CS. Participants were studied under smoking-abstinent and non-abstinent conditions to determine whether smoking cessation would exacerbate urges for caffeine in these smokers.
2. Methods
2.1 Participants
This study was conducted during a larger study that investigated the effects of a medication on smoking cue reactivity (Tidey et al., 2006); participants in the current study were tested under non-medicated conditions. Participants with schizophrenia or schizoaffective disorder (SS) and non-psychiatric control participants (CS) were recruited from the community using flyers and newspaper advertisements. All were at least 18 years old, had smoked between 20 and 50 cigarettes per day for the past year, had expired breath carbon monoxide levels of 18 ppm or more, were daily caffeine users, and had Fagerström Test for Nicotine Dependence (FTND) scores of 6 or more (Heatherton et al., 1991). The Structured Clinical Interview for DSM-IV (SCID; First et al., 1994) was used to confirm diagnoses of schizophrenia or schizoaffective disorder and rule out current Axis I disorders in control participants. Participants were excluded if they were pregnant or nursing, using alternative tobacco products; had breath alcohol levels of 0.005 g/l or more; had current substance abuse or dependence other than nicotine; were actively trying to quit smoking, were currently using medications known to affect cigarette smoking, or if the severity of their psychiatric symptoms would interfere with the completion of study procedures. Sixteen out of 18 (89%) of SS and 19 out of 25 (76%) of CS screened for this study met the daily caffeine use criterion and were enrolled; this difference was not significant (Χ2 (1, N=43) = 1.15, p = 0.28). All study procedures were approved by the institutional review boards of the medical center and university involved.
2.2 Individual Difference Measures
Information was collected on demographic variables, smoking histories and caffeine use histories. Current psychiatric symptoms for participants with schizophrenia or schizoaffective disorder were assessed using the Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987) and the Brief Psychiatric Rating Scale (BPRS; Overall and Gorham, 1962). Current depression was assessed in all participants, using the Hamilton Depression Rating Scale (HAM-D; Hamilton, 1960).
2.3 Cue Reactivity Assessment
Using a within-subjects design, responses to smoking cues were assessed under 5-hr smoking abstinent and non-abstinent conditions, with two replications of each condition, for a total of 4 study sessions. Participants arrived at the laboratory for each session at approximately 8 a.m. They were instructed to follow their typical smoking and caffeine use patterns prior to arrival at the laboratory. Upon arrival, participants provided breath samples for the assessment of carbon monoxide levels (Smokerlyzer, Bedfont Scientific Ltd., Kent, UK). For the next five hours, participants remained within the laboratory and were either permitted to smoke freely (non-abstinent condition) or were not allowed to smoke (abstinent condition). During this time, participants were allowed to read magazines and/or watch movies that were determined to contain no smoking cues. Participants were under continuous observation except for bathroom and lunch breaks, with CO monitoring to assure compliance with abstinence. Participants were not permitted to drink caffeinated beverages during lunch breaks.
At the end of this 5-hr period, breath CO levels were assessed, and the cue reactivity trial commenced as described previously (Tidey et al., 2005; 2008). Following a 10-minute relaxation period, urge for smoking was assessed (data not shown), and urge for caffeine was assessed using the question “How strong is your urge for a caffeinated beverage such as coffee, tea or cola right now?”, rated on a 100 mm visual analogue scale (VAS), with the anchors 0 = “no urge at all” and 100 = “strongest urge you’ve ever had”. Participants were then presented with the neutral cues (pencil, 25 mm × 65 mm eraser and a small pad of paper) on a covered tray. An audiotape instructed participants to lift the cover, look at and handle these cues. After 4 min had elapsed, participants were asked to complete the smoking and caffeine urge measures. The neutral cues were then replaced with the smoking cues (cigarette, lighter and ashtray) on a covered tray. Participants listened to audiotaped instructions to lift the cover and look at the cues. After 2 min elapsed, participants were asked to light the cigarette without inhaling. After 2 more min elapsed, participants were asked to extinguish the cigarette and to complete the smoking and caffeine urge measures. The smoking cue trial was then repeated to determine whether participants became habituated or sensitized to cues with repeated viewings. A research assistant monitored the sessions through a one-way mirror to ensure compliance with procedures.
2.4 Data analysis
Dependent variables were examined for distributional assumptions and collinearity. Sphericity was intact. Caffeine urge scores from the first and second smoking cues trials within each session were compared using Pearson Product-moment correlations. Since the two smoking cue trials were highly associated (r33 = .91, p < .001), the mean caffeine urge rating was entered into the analysis. Group comparisons on demographic, smoking history and caffeine history measures were conducted using one way ANOVAs for continuous variables and chi-square tests for categorical variables. Daily caffeine intake from coffee, tea and soda were calculated by querying product type, brand, brewing method and cup size. Caffeine content per cup was then calculated using standard values for coffee and tea (Barone and Roberts, 1996) and specific product information available online for soda (Center for Science in the Public Interest, 2007). As a manipulation check, a mixed-factor 2 × 2 ANOVA was used to examine the effects of Group (SS, CS) and Abstinence (Abstinent, Non-abstinent) on breath CO level after the 5-hr smoking and abstinence periods. Mixed-factor 2 × 2 × 2 ANOVAs were used to examine the effects of Group, Abstinence and Cue (neutral, smoking) on urge for a caffeinated beverage. In a second analysis, total daily caffeine intake was entered as a covariate given the trend toward a difference between the groups on this measure. Significant interactions were followed by simple effects tests. Differences were considered significant when p ≤.05. Effect sizes (η2) are also provided when p = .05 –.10, with η2 ≤ .05 for small, η2 = .06 – .13 for medium and η2 ≥ .14 for large effect sizes (Cohen, 1988). Analyses were conducted using SPSS 14.0 for Windows.
3. Results
3.1 Demographic and Clinical Characteristics
Fifteen SS and 18 CS completed the study. Demographic information, psychiatric symptom scores, smoking histories and caffeine use histories of participants are shown in Table 1. No demographic variable differed between groups. The SS group had higher depression scores (HAM-D) than the NCL group, (F (1, 29) = 14.20, p = .001), which was expected given the inclusion of participants with schizoaffective disorder within the SS sample. SS and CS did not differ in their reported daily total daily caffeine consumption, although the effect size was large (F (1, 31) = 1.51, p = .23, η2 = .21). The pattern of caffeinated beverage preference did not differ by group (Table 1). There was no increased frequency of extreme caffeine use (>200 mg/day) in the SS group compared to the CS group.
Table 1.
Demographic and Baseline Smoking Characteristics of Study Participants
| Control (n = 18) | Schizophrenia (n = 15) | |
|---|---|---|
| Female (%) | 22.2 | 40.0 |
| Age (years) | 46.1 (11.3) | 46.6 (6.4) |
| Education (years) | 12.7 (2.1) | 12.1 (1.4) |
| Race | ||
| European American (%) | 72 | 80 |
| African American (%) | 28 | 13 |
| Native American/Alaskan (%) | 0 | 0 |
| Multiracial (%) | 0 | 7 |
| Cigarettes per day | 26.4 (7.8) | 26.8 (10.6) |
| FTND | 7.3 (1.7) | 7.2 (1.7) |
| Breath CO (ppm) | 22.4 (10.8) | 26.5 (13.1) |
| Daily total caffeine consumption (mg) | 472.4 (422.3) | 664.8 (478.1) |
| Daily coffee consumption (mg) | 438.5 (433.0) | 617.4 (464.2) |
| Daily tea consumption (mg) | 38.3 (47.6) | 94.0 (0) |
| Daily caffeinated soda consumption (mg) | 52.3 (65.4) | 88.1 (52.2) |
| Daily coffee consumers (%) | 83.3 | 100 |
| Daily tea consumers (%) | 16.7 | 6.7 |
| Daily caffeinated soda consumers (%) | 33.3 | 46.7 |
| Daily consumers of more than one caffeinated beverage (%) | 27.8 | 53.3 |
| Age at first caffeine consumption | 11.1 (4.8) | 13.9 (8.8) |
| Age of daily caffeine consumption | 17.2 (8.4) | 16.5 (9.7) |
| Years of caffeine consumption | 29.1 (14.3) | 30.0 (9.8) |
| HAM-D total score | 2.7 (2.5) | 8.8 (6.2)** |
| PANSS positive scale | 11.7 (4.3) | |
| PANSS negative scale | 12.8 (4.8) | |
| PANSS general psychopathology scale | 26.1 (7.9) | |
| Antipsychotic medication | ||
| Typical antipsychotics (%) | 26.7 | |
| Atypical (%) | 53.3 | |
| Antidepressant medication (%) | 60.0 | |
| Antianxiety medication (%) | 20.0 | |
Abbreviations: Fagerström Test of Nicotine Dependence; CO, carbon monoxide; HAM-D, Hamilton Depression Rating Scale; PANSS, Positive and Negative Syndrome Scale.
p < .05
p < .01
Caffeine use was significantly correlated with the number of cigarettes smoked per day across groups (r33 = .50, p < .01). While this correlation remained significant within the SS sample (r15 = .72, p < .01), the correlation between caffeine use and cigarette smoking was not significant in the CS sample (r18 = .25, p = .32).
3.2 Effects of Smoking Abstinence and Smoking Cues
There was a significant main effect of smoking abstinence on breath CO level (F (1, 22) = 57.13, p < .001). Abstinence decreased breath CO levels in both groups (non-abstinent: 21.7 ± 2.0 [M ± SD]; abstinent: 9.4 ± 0.8). There was no main effect of Group or Group × Abstinence interaction on this variable.
A main effect of Group on caffeine urge approached significance, with means indicating that SS tended to report stronger urges for caffeine across cue types and abstinence states than CS (SS: 50.1 ± 8.1; CS: 29.0 ± 7.4; F (1, 31) = 3.72, p = .06). There was a main effect of Abstinence on caffeine urge (F (1, 31) = 8.07, p < .01), with participants reporting stronger urges for caffeine when smoking-abstinent (43.3 ± 5.9) compared to when they were non-abstinent (35.8 ± 5.4). There was a main effect of Cue type and a significant Cue type × Group interaction on caffeine urge (F (1, 31) = 11.42, p <.01; F (1, 31) = 4.24, p < .05; respectively). Post-hoc tests indicated that smoking cues increased caffeine urges in SS but not in CS.
Because daily caffeine consumption tended to be higher in the SS group, these data were reanalyzed with daily caffeine consumption included as a covariate. In these analyses, the main effect of Group on caffeine urge was not significant, and the effect of smoking abstinence on caffeine urge was reduced to trend-level (F (1, 30) = 2.91, p = .10, η2 = .09). However, the main effect of Cue type and the Cue type × Group interaction on caffeine urge remained significant (F (1, 30) = 4.95, p <.05; F (1, 30) = 4.14, p = .05, respectively). As shown in Figure 1, smoking cues increased caffeine urges in SS (t (14) = 2.72, p < .05) but not in CS. There were no other significant interactions on caffeine urge ratings.
Figure 1.
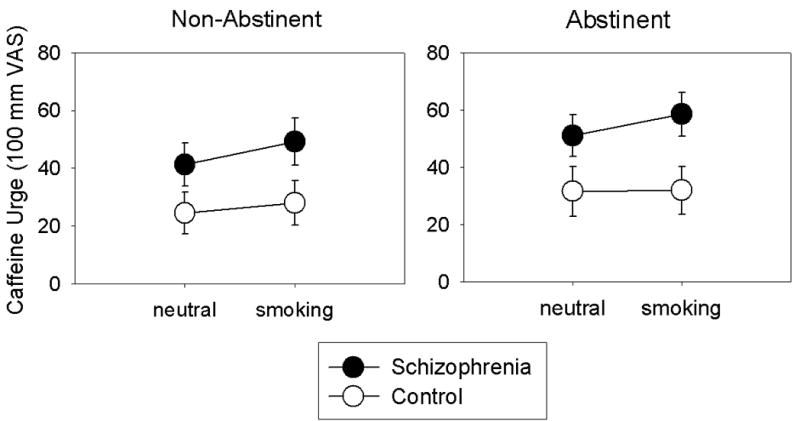
Effects of neutral and smoking cues on urges for caffeine in smokers with schizophrenia (n = 15) and control smokers (n = 18) under non-abstinent and 5-hr smoking abstinent conditions. Data points represent M ± SEM.
4. Discussion
The results of this study indicate that SS were more sensitive to the effects of smoking cues on urges for caffeinated beverages, an effect that remained significant after controlling for group differences in caffeine intake. To our knowledge, this study is the first to examine cross-cue reactivity between cigarettes and caffeinated beverages, and the first to report that smoking cues increase caffeine urges in smokers with schizophrenia. Cross-cue reactivity is thought to arise through associative learning when individuals have a history of using two substances together (Drobes et al., 2002; Rohsenow et al., 1997). Thus, these data suggest that co-use of cigarettes and caffeinated beverages may be more common among SS than among CS. In turn, their propensity to experience urges for caffeinated beverages while in the presence of smoking-related stimuli may be one behavioral mechanism that contributes to higher caffeine consumption in SS. This hypothesis is supported by the observation of a significant correlation between average daily smoking rate and daily caffeine ingestion in the SS group, which has also been reported by others (Strassnig et al., 2006). This hypothesis could be further investigated with ecological momentary assessment techniques to obtain real time measures of smoking and caffeine consumption in SS and CS.
Another plausible explanation for the increased cross-cue reactivity in SS is that the neuropathology associated with schizophrenia, specifically hyperactivity of the mesolimbic DA system or inadequate cortical and hippocampal control over subcortical DA, may confer increased sensitivity to drug-associated cues (Chambers et al., 2001). A report of stronger cocaine cue reactivity in cocaine users with schizophrenia compared to those without schizophrenia supports this suggestion (Smelson et al., 2002). However, studies to date have not found smokers with schizophrenia to be more sensitive than those without schizophrenia to the effects of smoking cues on smoking urges (Fonder et al. 2005; Tidey et al., 2005, 2008). Thus, evidence supporting the hypothesis that schizophrenia is associated with heightened sensitivity to drug-associated cues is currently equivocal. This could be explored further using a cue reactivity design that incorporates functional magnetic resonance imaging (e.g., McClernon and Gilbert, 2004). Abstinence from smoking did not appreciably increase caffeine urges in either group, which contrasts with the robust effects of 5-hr smoking abstinence on smoking urges in SS and CS (Tidey et al., 2005, 2008), and suggests that urges for caffeine would not increase during the early phase of a smoking cessation attempt.
SS tended to consume more caffeine than CS in this study. Although this difference was not statistically significant, this is likely due to the small sample sizes in this study as the effect size for this group comparison was large. High levels of caffeine consumption in people with schizophrenia have been reported previously by others (e.g., Benson et al., 1986; Gurpegui et al., 2006; Roick et al., 2007; Zaslove et al., 1991). Consistent with these reports of increased caffeine consumption, a recent study found that SS had higher serum caffeine levels than CS after controlling for smoking rate, antipsychotic medication type and dose (Gandhi et al., 2008). Although preliminary, these data are not indicative of a differential rate of caffeine metabolism in smokers with and without schizophrenia. As deleterious behavioral and health consequences of high caffeine consumption in people with schizophrenia have been reported (reviewed in de Leon et al., 2004; Hughes et al., 1998; Williams and Gandhi, 2008), this is an important clinical issue that merits further research and intervention.
This study has at least one limitation. We did not assess time since last cigarette or caffeine consumption upon session arrival. Because of the intensity of the smoking manipulation (i.e., 5-hr abstinence or ad libitum smoking) and because all participants were abstinent from caffeine for at least 5 hours prior to cue reactivity assessment, individual differences in time since last cigarette or caffeine consumption at session arrival are unlikely to have systematically affected the study results. However, future studies could include measurement of serum caffeine levels to assess and statistically control for recent caffeine use. Furthermore, we did not include an assessment of urge for non-caffeinated beverages, so it is possible that smoking cues increased urges for beverages in general rather than specific urges for caffeine. Future studies should include this question as a control. Future studies could also include a behavioral measure of caffeine consumption, such as latency to consume a caffeinated beverage or frequency of choice for a caffeinated beverage versus a non-caffeinated beverage, to validate the caffeine urge measure. Nevertheless, the results of this study are significant because they suggest a mechanism that may underlie the frequent clinical observation that high levels of caffeine consumption are common among people with schizophrenia.
Acknowledgments
5. Author Disclosure
Funding for this study was provided by NIDA grant R01-DA14002 to J.W.T.; NIDA had no other role in the design or conduct of this study. This study was conducted at the Providence Veterans Affairs Medical Center and the Brown University Center for Alcohol and Addiction Studies, Providence, RI, USA. Preliminary data from this study were presented at the 2008 annual meeting of the College on Problems of Drug Dependence.
A.A. and J.W.T. designed the study and wrote the protocol, A.A. collected and managed the data and C.A. performed the data analysis. All authors contributed to the manuscript and approved the final draft. No author has any conflict of interest with this research.
We thank Gary Kaplan, M.D., for encouraging us to study caffeine consumption in patients with schizophrenia. We thank Laura Dionne for assistance with data management, and the individuals who participated in this study for their contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barone JJ, Roberts HR. Caffeine consumption. Fd Chem Tox. 1996;34:119–129. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- Benson JI, David JJ. Coffee eating in chronic schizophrenic patients. Am J Psychiatry. 1986;143:940–941. doi: 10.1176/ajp.143.7.940b. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327 – 340. [PubMed] [Google Scholar]
- Center for Science in the Public Interest. [Retrieved August 25, 2008];Caffeine content of food and drugs. 2007 from http://www.cspinet.org/new/cafchart.htm.
- Chait LD, Griffiths RR. Effects of caffeine on cigarette smoking and subjective response. Clin Pharmacol Ther. 1983;34:612–622. doi: 10.1038/clpt.1983.223. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Colby SM, Rohsenow DJ, Monti PM, Gwaltney CJ, Gulliver SB, Abrams DB, Niaura RS, Sirota AD. Effects of tobacco deprivation on alcohol cue reactivity and drinking among young adults. Addict Behavi. 2004;29:879–892. doi: 10.1016/j.addbeh.2004.03.002. [DOI] [PubMed] [Google Scholar]
- de Leon J. Atypical antipsychotic dosing: the effect of smoking and caffeine. Psychiatr Serv. 2004;55:491–493. doi: 10.1176/appi.ps.55.5.491. [DOI] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76:135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- de Leon J, Verghese C, Tracy JI, Josiassen RC, Simpson GM. Polydipsia and water intoxication in psychiatric patients: a review of the epidemiological literature. Biol Psychiatry. 1994;35:408–419. doi: 10.1016/0006-3223(94)90008-6. [DOI] [PubMed] [Google Scholar]
- de Leon J, Tracy J, McCann E, McGory A, Diaz F. Schizophrenia and tobacco smoking: A replication study in another US psychiatric hospital. Schizophr Res. 2002;56:55–65. doi: 10.1016/s0920-9964(01)00192-x. [DOI] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ, Rogers T, Browne D, Dinsmore L, Ghosheh O, Dwoskin LP, Crooks PA. A pilot study of plasma caffeine concentrations in a US sample of smoker and non-smoker volunteers. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:165–171. doi: 10.1016/s0278-5846(02)00348-2. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W. Cannabis and psychosis. Curr Psychiatry Rep. 2002;4:191–196. doi: 10.1007/s11920-002-0026-5. [DOI] [PubMed] [Google Scholar]
- Drobes DJ. Cue reactivity in alcohol and tobacco dependence. Alcohol Clin Exp Res. 2002;26:1928–1929. doi: 10.1097/01.ALC.0000040983.23182.3A. [DOI] [PubMed] [Google Scholar]
- Duka T, Tasker R, Russell K, Stephens DN. Discriminative stimulus properties of nicotine at low doses: the effects of caffeine preload. Behav Pharmacol. 1998;9:219–229. [PubMed] [Google Scholar]
- Emurian HH, Nellis MJ, Brady JV, Ray RL. Event time-series relationship between cigarette smoking and coffee drinking. Addict Behav. 1982;7:441–444. doi: 10.1016/0306-4603(82)90016-8. [DOI] [PubMed] [Google Scholar]
- Eriksson A, Tengstrom A, Hodgins S. Typologies of alcohol use disorders among men with schizophrenic disorders. Addict Behav. 2007;32:1146–1163. doi: 10.1016/j.addbeh.2006.08.003. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- Fonder MA, Sacco KA, Termine A, Boland BS, Seyal AA, Dudas MM, Vessicchio JC, George TP. Smoking cue reactivity in schizophrenia: Effects of a nicotinic receptor antagonist. Biol Psychiatry. 2005;57:802–808. doi: 10.1016/j.biopsych.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Gandhi KK, Williams JM, Galazyn M, Benowitz N. Higher serum caffeine in smokers with schizophrenia as compared to controls; Presented at the 14th annual meeting of the Society for Research on Nicotine and Tobacco (SRNT); Portland, OR. 2008. Feb, [Google Scholar]
- Gurpegui M, Aguilar MC, Martinez-Ortega JM, Jurado D, Diaz FJ, Quintana HM, de Leon J. Fewer but heavier caffeine consumers in schizophrenia: A case-control study. Schizophr Bull. 2006;86:276–283. doi: 10.1016/j.schres.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Gurpegui M, Jurado D, Luna JD, Fernandez-Molina C, Moreno-Abril O, Galvez R. Personality traits associated with caffeine intake and smoking. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:997–1005. doi: 10.1016/j.pnpbp.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatr. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell CF, Kennedy DO, Wesness KA, Scholey AB. Cognitive and mood improvements of caffeine in habitual consumers and habitual non-consumers of caffeine. Psychopharmacology. 2005;179:813–825. doi: 10.1007/s00213-004-2104-3. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, McHugh P, Holtzman S. Caffeine and schizophrenia. Psychiatr Serv. 1998;49:1415–1417. doi: 10.1176/ps.49.11.1415. [DOI] [PubMed] [Google Scholar]
- Istvan J, Matarazzo JD. Tobacco, alcohol and caffeine use: a review of their interrelationships. Psychol Bull. 1984;95:301–326. [PubMed] [Google Scholar]
- Jones HE, Griffiths RR. Oral caffeine maintenance potentiates the reinforcing and stimulant subjective effects of intravenous nicotine in cigarette smokers. Psychopharmacology. 2003;165:280–290. doi: 10.1007/s00213-002-1262-4. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis and nicotine use from early adolescence to middle adulthood. Arch Gen Psychiatry. 2008;65:674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Guey LT, Bromet EJ, Schwartz JE. Smoking in schizophrenia: diagnostic specificity, symptom correlates, and illness severity. Schizophr Bull. 2008 doi: 10.1093/schbul/sbn066. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WR, Green SB, Epstein LH, Rogers CM, McCoy JF. Coffee drinking and cigarette smoking: II. Coffee, urinary pH and cigarette smoking behavior. Addict Behav. 1980;5:395–400. doi: 10.1016/0306-4603(80)90013-1. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Gilbert DG. Human functional neuroimaging in nicotine and tobacco research: basics, background and beyond. Nicotine Tob Res. 2004;6:941–959. doi: 10.1080/14622200412331337394. [DOI] [PubMed] [Google Scholar]
- Nellis MJ, Emurian HH, Brady JV, Ray RL. Behavior analysis of cigarette smoking. Pavlov J Biol Sci. 1982;17:140–149. doi: 10.1007/BF03001208. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- Perkins KA, Sexton JE, Stiller RL, Fonte C, DiMarco A, Goettler J, Scierka A. Subjective and cardiovascular responses to nicotine combined with caffeine during rest and casual activity. Psychopharmacology. 1994;113:438–444. doi: 10.1007/BF02245220. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Stolinski A, Blakesley-Ball R, Wilson AS. The influence of caffeine on nicotine’s discriminative stimulus, subjective and reinforcing effects. Exp Clin Psychopharmacol. 2005;13:275–281. doi: 10.1037/1064-1297.13.4.275. [DOI] [PubMed] [Google Scholar]
- Rihs M, Mueller C, Baumann P. Caffeine consumption in hospitalized psychiatric patients. Eur Arch Psychiatry Clin Neurosci. 1996;246:83–92. doi: 10.1007/BF02274898. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Colby SM, Gulliver SB, Sirota AD, Niaura RS, Abrams DB. Effects of alcohol cues on smoking urges and topography among alcoholic men. Alcohol Clin Exp Res. 1997;21:101–107. [PubMed] [Google Scholar]
- Schneier FR, Siris SG. A review of psychoactive substance use and abuse in schizophrenia. Patterns of drug choice. J Nerv Ment Dis. 1987;175:641–652. doi: 10.1097/00005053-198711000-00001. [DOI] [PubMed] [Google Scholar]
- Shaner A, Eckman TA, Roberts LJ, Wilkins JN, Tucker DE, Tsuang JW, Mintz J. Disability income, cocaine use, and repeated hospitalization among schizophrenic cocaine abusers – a government-sponsored revolving door? N Engl J Med. 1995;333:777–783. doi: 10.1056/NEJM199509213331207. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Swanner LS, Yasar S, Goldberg SR. Chronic caffeine exposure potentiates nicotine self-administration in rats. Psychopharmacology. 1999;142:327–333. doi: 10.1007/s002130050896. [DOI] [PubMed] [Google Scholar]
- Smelson DA, Losonczy MF, Kilker C, Starosta A, Kind J, Williams J, Ziedonis D. An analysis of cue reactivity among persons with and without schizophrenia who are addicted to cocaine. Psychiatr Serv. 2002;53:1612–1616. doi: 10.1176/appi.ps.53.12.1612. [DOI] [PubMed] [Google Scholar]
- Strassnig M, Brar JS, Ganguli R. Increased caffeine and nicotine consumption in community-dwelling patients with schizophrenia. Schizophr Res. 2006;86:269–275. doi: 10.1016/j.schres.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Tanda G, Goldberg SR. Alteration of behavioral effects of nicotine by chronic caffeine exposure. Pharmacol Biochem Behav. 2000;66:47–64. doi: 10.1016/s0091-3057(00)00234-3. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM. Subjective and physiological responses to smoking cues in smokers with schizophrenia. Nicotine Tob Res. 2005;7:421–429. doi: 10.1080/14622200500125724. [DOI] [PubMed] [Google Scholar]
- Tidey J, Rohsenow D, Kaplan G, Swift R. Effects of smoking cues, acute abstinence and bupropion in smokers with schizophrenia and non-psychiatric controls; Presented at the 12th annual meeting of the Society for Research on Nicotine and Tobacco; Orlando, FL. 2006. Mar, [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Adolfo AB. Effects of smoking abstinence, smoking cues and nicotine replacement in smokers with schizophrenia and controls. Nicotine Tob Res. 2008;10:1047–1056. doi: 10.1080/14622200802097373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Gandhi KK. Use of caffeine and nicotine in people with schizophrenia. Current Drug Abuse Reviews. 2008;1(2):155–161. doi: 10.2174/1874473710801020155. [DOI] [PubMed] [Google Scholar]
- Winklbaur B, Ebner N, Sachs G, Thau K, Fischer G. Substance abuse in patients with schizophrenia. Dialogues Clin Neurosci. 2006;8:37–43. doi: 10.31887/DCNS.2006.8.1/bwinklbaur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaslove MO, Russell RL, Ross E. Effect of caffeine intake on psychotic in-patients. Br J Psychiatry. 1991;159:565–567. doi: 10.1192/bjp.159.4.565. [DOI] [PubMed] [Google Scholar]


