Abstract
S-Ribosylhomocysteinase (LuxS) catalyzes the cleavage of the thioether bond of S-ribosylhomocysteine (SRH) to produce homocysteine and 4,5-dihydroxy-2,3-pentanedione (DPD), which is the precursor of type 2 autoinducer for bacterial cell-cell communication. In this work, we have synthesized several SRH analogues modified at the ribose C3 position as potential inhibitors of LuxS. While removal or methylation of the C3-OH resulted in simple competitive inhibitors of moderate potency, inversion of the C3 stereochemistry or substitution of fluorine for C3-OH resulted in slow-binding inhibitors of improved potency. The most potent inhibitor showed a KI* value of 0.43 µM.
Keywords: Enzyme inhibition, Fluoro sugars, LuxS enzyme, S-Ribosylhomocysteine
Introduction
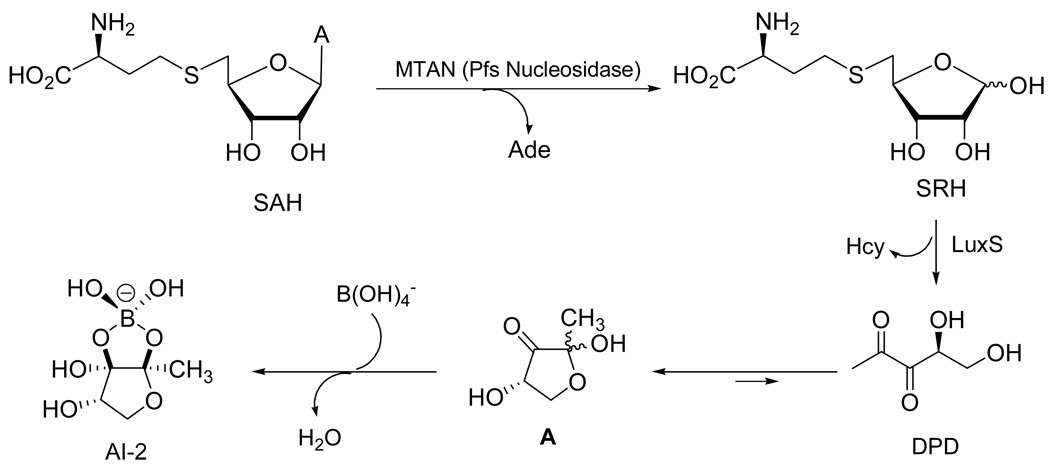
The bacterial enzyme 5'-methylthioadenosine/S-adenosylhomocysteine (SAH) nucleosidase (MTAN, EC 3.2.2.9) catalyzes the depurination of SAH to form adenine and S-ribosyl-L-homocysteine (SRH) (Figure 1).1 Encoded by the pfs gene, MTAN is involved in several bacterial processes including polyamine biosynthesis, quorum sensing (QS), methyl-transfer reactions, and adenine and methionine salvage.2 SRH is subsequently converted to L-homocysteine and 4,5-dihydroxy-2,3-pentanedione (DPD) by the enzyme LuxS (or S-ribosylhomocysteinase).3 DPD is in equilibrium with its cyclic hemiketal A and forms several QS molecules, which function as bacterial interspecies signaling molecules called autoinducers 2 (AI-2), and are detected by a wide range of bacteria.4–6 The (2S,4S)-isomer of the hemiketal A undergoes complexation with borate to form a furanosyl borate diester as a distinct AI-2.7 Recent chemical synthesis of the unstable DPD and their analogues has made it possible to assess the complexation properties of DPD with borate and provided DPD samples for investigation of AI-2-regulated processes.8–15 QS regulates many crucial bacterial functions such as symbiosis, virulence, antibiotic production, biofilm formation.16–19 Therefore, proteins involved in QS including LuxS are being pursued as targets for designing novel antibacterial agents.
Figure 1.
Enzymatic conversion of SAH by MTAN and LuxS: Biosynthetic pathway to AI-2.
LuxS is a small metalloenzyme containing a Fe2+ ion coordinated by His-54, His-58, Cys-126, and a water molecule. The native enzyme is unstable under aerobic conditions, but substitution of Co2+ for Fe2+ gives a highly stable variant with wild-type catalytic activity.20,21 In the proposed catalytic mechanism of LuxS, the metal ion acts as a Lewis acid and catalyzes two consecutive aldose-ketose (C1 → C2) and ketose-ketose (C2 → C3) isomerization steps and the β-elimination of Hcy from a 3-keto intermediate to give DPD.22–24 Cleavage of the C5–S thioether bond of SRH by LuxS is mechanistically distinct from the reversible reaction catalyzed by SAH hydrolase, which cleaves the equivalent thioether bond in SAH. The latter process is initiated by oxidation of the C3' alcohol of SAH with an NAD+ cofactor to give a 3'-keto intermediate that undergoes β-elimination of Hcy.25,26
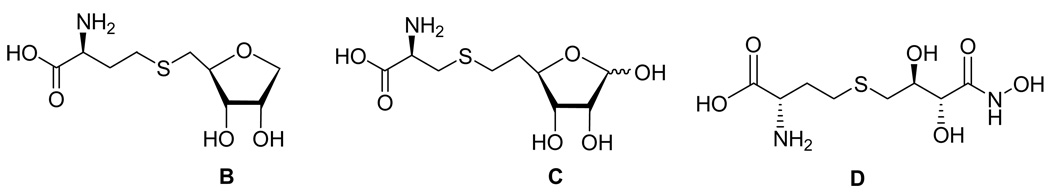
Recently, Zhou and coworkers designed two substrate analogues, the S-(anhydroribosyl)-L-homocysteine (B) and S-(homoribosyl)-L-cysteine (C) compounds (Figure 2). The initial mechanistic step was prevented by B, and the final step by C.27 Pei and coworkers28 prepared a series of structural analogues of the enediolate intermediates, in which the unstable enediolate moiety was replaced with a stable hydroxamate group. Among them, stable isostere D inhibited LuxS at submicromolar concentrations (KI = 0.72 µM). The ribosyl and xylosylhomocysteines in which the carbon-5 and sulfur atoms were replaced by a vinyl or (fluoro)vinyl unit did not show significant inhibition of LuxS.29 We now describe the synthesis and evaluation of several carbon-3 modified SRH analogues that lack a hydroxyl group which can be converted into an enol. These compounds act as mechanism-based inhibitors of LuxS and could impede the production of autoinducers of type 2, and disrupt quorum sensing and cell-cell communication in bacteria.
Figure 2.
LuxS inhibitors
Results and Discussion
Chemistry
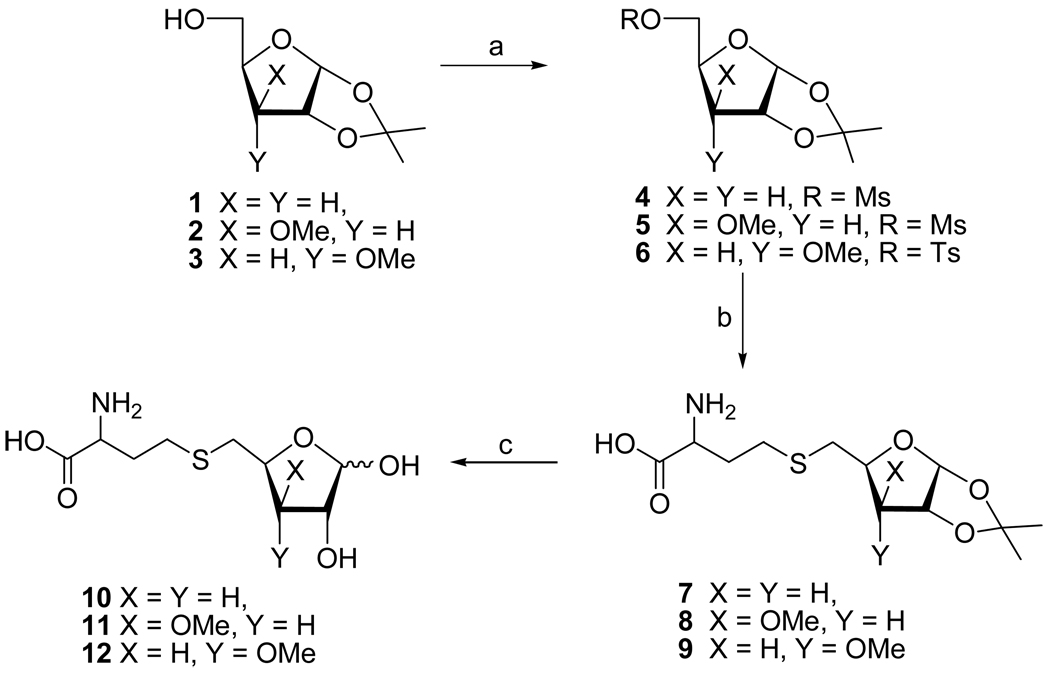
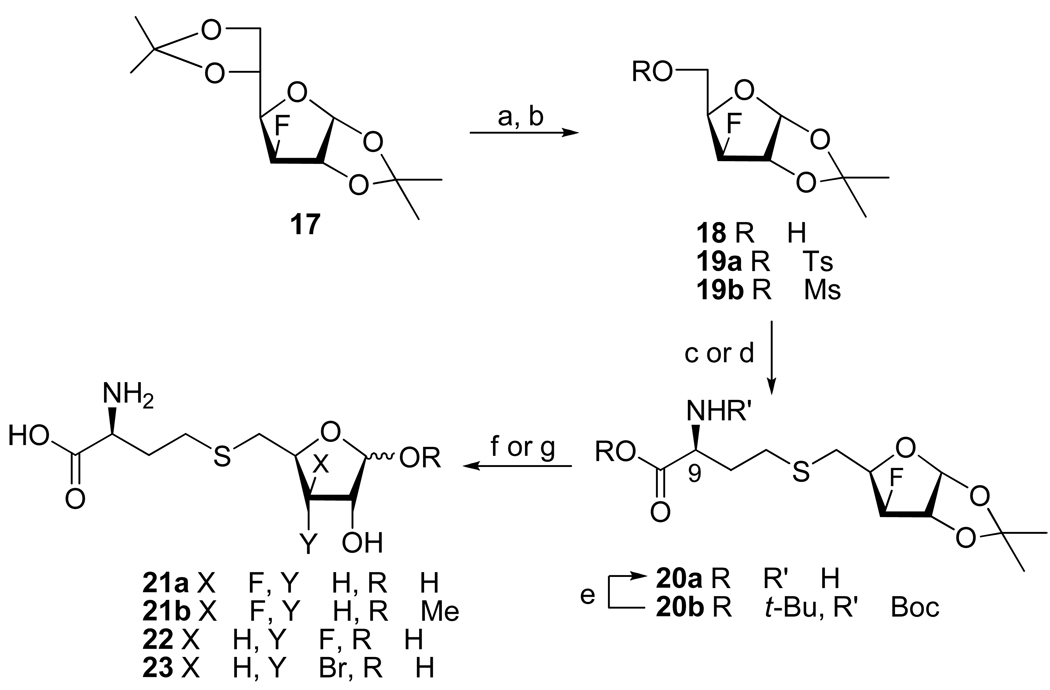
We initially targeted 3-deoxy-SRH analogue 10 because it cannot undergo the second enolization step to produce the 3-keto intermediate.3,22 Treatment of diacetone 3-deoxyglucose30 with periodic acid selectively removed the 5,6-O-isopropylidene group and oxidatively cleaved the resulting vicinal 5,6-diol31 to give the corresponding 5-aldehyde, which was reduced with NaBH4 to give 3-deoxy furanoside 1 (Scheme 1). Mesylation at C5, displacement with thiolate derived from D/L-homocysteine,32 and treatment with aqueous trifluoroacetic acid gave the desired S-(3-deoxyribosyl)homocysteine 10 (α/β, 1:3; 25% from 1).
Scheme 1.
Reagents: (a) MsCl or TsCl/Et3N; (b) Hcy/NaOH/MeOH/H2O; (c) TFA/H2O
Next, the 3-O-methyl xylo- (11) and ribofuranosyl analogues (12) were prepared as potential LuxS inhibitors, because the 3-methoxy group was expected to prevent the second enolization step as well. We were also interested to see whether the LuxS active site can tolerate both R and S epimers at the C3 position. Thus, mesylation of l,2-O-isopropylidene-3-O-methyl-α-D-xylofuranose 2 afforded ester 5. Nucleophilic displacement with the thiolate group of D/L-homocysteine afforded thioether 8. Deprotection (TFA/H2O) and purification by reversed-phase HPLC gave S-(3-O-methylxylosyl)homocysteine 11 as a mixture of two anomers (α/β, 1:1; 50%). S-(3-O-Methylribosyl)homocysteine 12 (α/β, 3:7) was similarly prepared from the corresponding ribose 3, except that the C5-OH group was activated by conversion into 5-O-tosylate 6 (instead of 5-O-mesylate) because treatment of the latter with Hcy/NaOH led to the hydrolysis of the mesylate and recovery of alcohol 3.
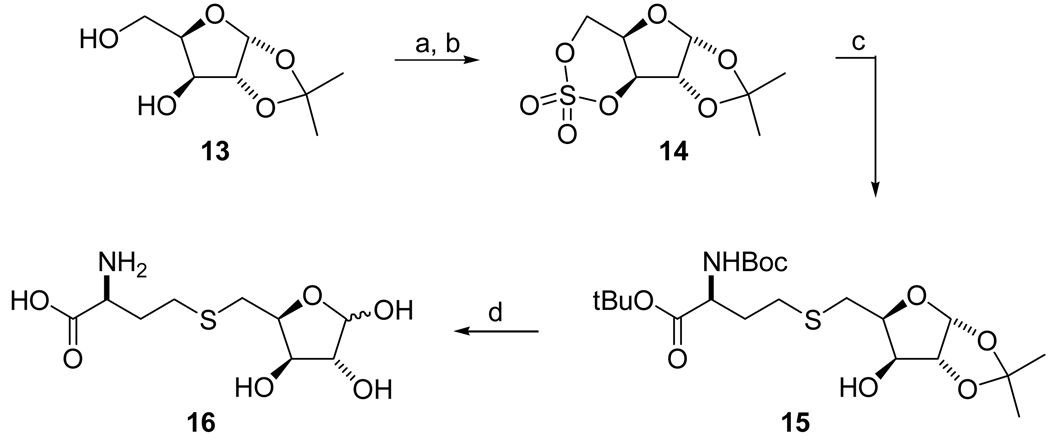
S-Xylosylhomocysteine (SXH, compound 16) was synthesized from 1,2-isopropylidene-α-D-xylofuranose 1333 (Scheme 2). The diol of 13 was converted into cyclic sulfate 14 by treatment with thionyl chloride in the presence of Et3N in DCM and followed by oxidation of the resulting sulfite with RuCl3 and NaIO4. The cyclic sulfate 14 was readily opened22 with the protected L-homocysteine in the presence of BuLi in DMF to give desired thioether 15, which was converted into the target compound SXH 16 by treatment with TFA.
Scheme 2.
Reagents: (a) SOCl2/Et3N/DCM/−78 °C – RT; (b) RuCl3/NaIO4/CCl4/CH3CN/H2O/0 °C – RT; (c) (i) BocNH-CH(CH2CH2SH)-CO2t-Bu/BuLi/DMF/0 °C – RT, (ii) THF/H2SO4/H2O/0 °C; (d) TFA/H2O/0 °C - RT.
S-(3-Deoxy-3-fluoroxylosyl)homocysteine 21a and the corresponding glycoside 21b were prepared from the readily available diacetone 3-deoxy-3-fluoroglucose 17.34 Thus, sequential treatment of 17 with H5IO6 and NaBH4 31 gave 3-deoxy-3-fluoro-l,2-O-isopropylidene-α-D-xylofuranose (18, 60%), which was then converted into 5-O-mesylate 19b (Scheme 3). Displacement of the mesylate group with thiolate generated from N-Boc-L-Hcy-CO2t-Bu (LDA/DMF) gave thioether 20b in 92% yield. It is noteworthy that the use of water soluble tris(2-carboxyethyl)phosphine for the generation of the protected homocysteine free thiol from N,N'-di(tert-butoxycarbonyl)-L-homocystine di(tert-butyl) ester not only improved the reaction yield but also simplified the purification process, as compared to the more commonly used tributyl-22 and triethylphoshines27 for the reduction of homocystine analogues. Deprotection (TFA followed by TFA/H2O) of 20b gave S-(3-deoxy-3-fluoroxylosyl)homo-cysteine 21a, which showed two sets of peaks at the 19F NMR spectrum at δ −200.55 (ddd, J = 12.6, 28.0, 50.6 Hz, 0.6F; β) and −202.41 (ddd, J = 18.7, 25.4, 52.1 Hz, 0.4F; α). Treatment of thioether 20b with BCl3 at −50 °C followed by quenching of the reaction mixture with MeOH resulted in the removal of all protection groups and the formation of methyl glycoside 21b as a 1:1.1 mixture of α/β anomers (29%) as well as small amounts of 21a (7%). Displacement of the tosylate group from 19a with D/L-homocysteinate thiolate produced 20a (9R/S, 1:1) and subsequent acid-catalyzed removal of the acetonide group afforded 21a (9R/S, 1:1; α/β, 2:3), which showed four sets of peaks at the 19F NMR spectrum.
Scheme 3.
Reagents: (a) (i) H5IO6/EtOAc, (ii) NaBH4/EtOH; (b) TsCl/C5H5N or MsCl/Et3N/CH2Cl2; (c) Hcy/NaOH/MeOH/H2O; (d) BocNHCH(CHCHSH)CO2t-Bu/LDA/DMF; (e) TFA; (f) TFA/H2O; (g) BCl3/CH2Cl2/MeOH.
Synthesis of 3-deoxy-3-fluoro-SRH 22 and 3-bromo-3-deoxy-SRH analogue 23 have recently been described.35
Inhibition of LuxS
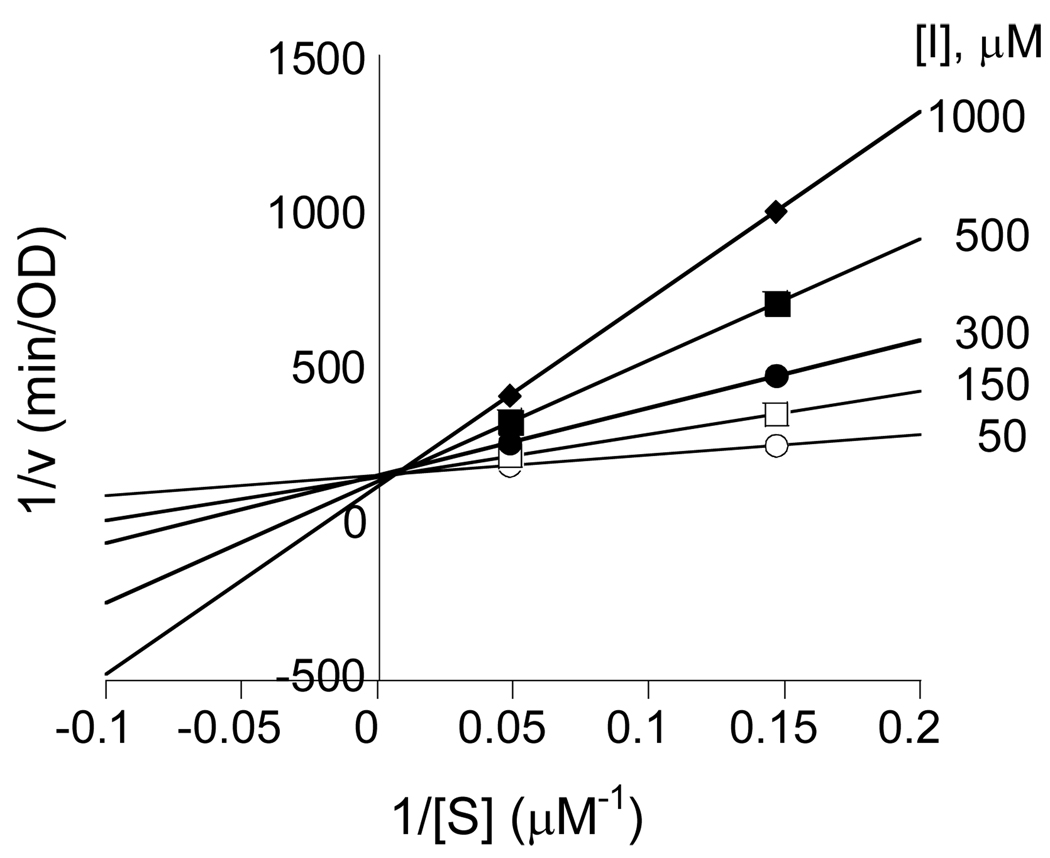
The SRH analogues 10–12, 16, 21a, and 21b were evaluated as potential inhibitors of Bacillus subtilis LuxS. Compound 10 acted as a simple, competitive inhibitor of moderate potency, with a KI value of 55 ± 6 µM (Table 1 and Figure 3). We also tested whether compound 10 can act as a substrate of LuxS. In particular, we were interested in whether it could be converted into the corresponding 2-ketone intermediate in the LuxS reaction. Since the intermediate cannot be converted into DPD product due to its lack of the 3-hydroxyl group, this would provide an alternative substrate to study the detailed mechanism of the first catalytic step. The 13C NMR spectrum of an overnight reaction mixture of 10 and LuxS failed to show any signal in the δ 180–230 region, indicating that no significant amount of the 2-keto intermediate was generated under the equilibrium conditions. Unfortunately, this result does not reveal whether removal of the C3-OH prevented the formation of the 2-ketone intermediate or simply shifted the equilibrium position between the substrate and the 2-ketone intermediate. Like the 3-deoxy analogue 10, the 3-methoxy analogues 11 and 12 also acted as competitive inhibitors, with KI values of 66 and 42 µM, respectively, against BsLuxS.
Table 1.
Inhibition constants of C3-modified SRH analogues against Bacillus subtilis LuxSa
| Compound | KI (µM) | KI* (µM) |
|---|---|---|
| 10 | 55 ± 6 | - |
| 11 | 66 ± 10 | - |
| 12 | 42 ± 8 | - |
| 16 | 4.2 ± 1.2 | 0.43 ± 0.04 |
| 21a | 7.7 ± 1.3 | 2.8 ± 0.3 |
| 21b | 42 ± 1 | 8.8 ± 1.6 |
| 22 | 10.6 ± 2.4 | 0.70 ± 0.26 |
| 23 | 7.9 ± 1.6 | 1.4 ± 0.4 |
Inhibition constants for compounds 10–12 were determined for D/L-isomers of Hcy (9R/S) and for the remaining compounds for pure L-form of Hcy (9S).
Figure 3.
Lineweaver-Burk plot showing competitive inhibition of 3-deoxy-SRH 10 against Co2+-substituted BsLuxS.
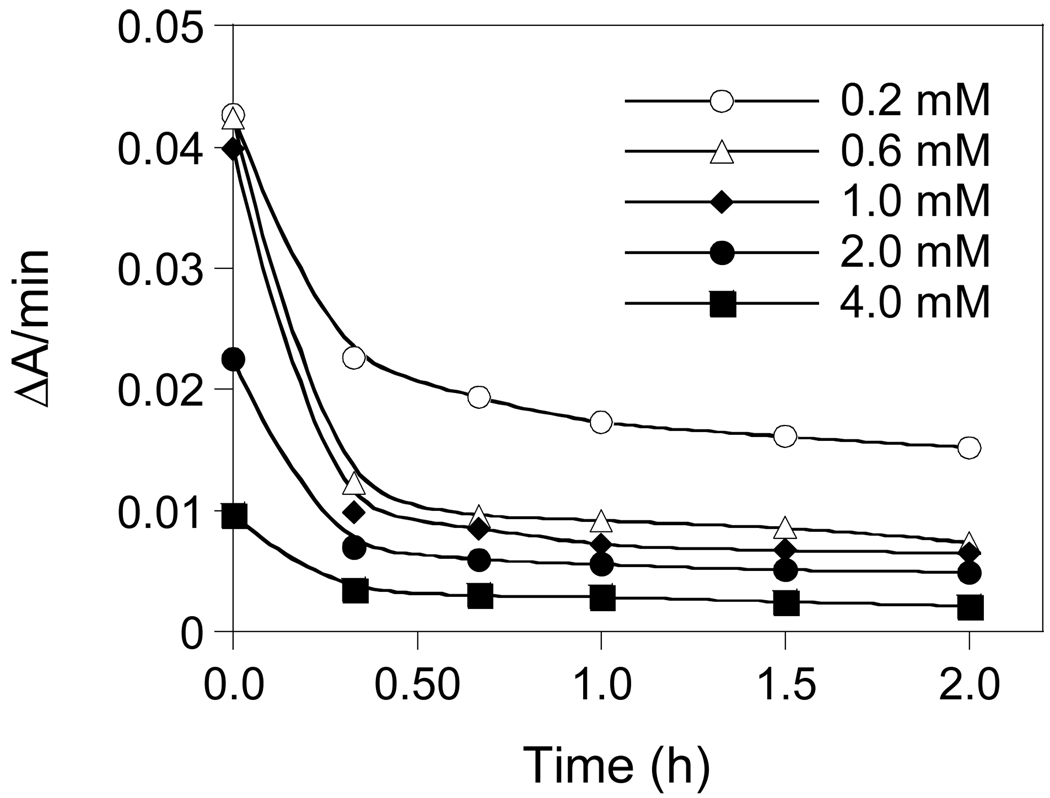
On the other hand, compounds 16, 21a, and 21b all exhibited time-dependent inhibition of LuxS. Figure 4 shows the example of inhibition of Vibrio harveyi LuxS (VhLuxS) by compound 21a. Preincubation of LuxS with the inhibitor resulted in gradual loss of LuxS activity with time; the activity decrease continued for ~60 min, when there was no further change in the residual activity. The compounds were also incubated with BsLuxS and the reaction mixtures were analyzed by electrospray ionization mass spectrometry to detect any covalent adduct between the enzyme and the inhibitors. We only observed signals that correspond to the unmodified BsLuxS protein, indicating that compounds 16, 21a, and 21b do not covalently modify the LuxS protein. Thus, these compounds act as slow-binding inhibitors of LuxS and their inhibition kinetics may be described by equation
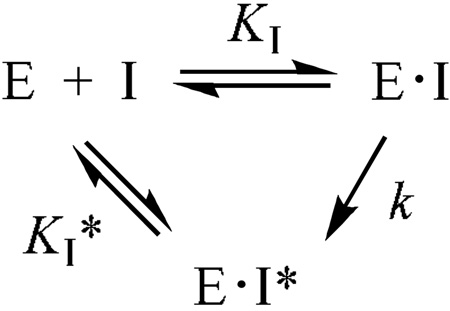 |
where E·I represents the initial enzyme-inhibitor complex, E·I* is the final, tighter enzyme-inhibitor complex, KI is the equilibrium constant for the formation of the initial E•I complex, k is the rate constant for the conversion of the E•I complex to the tighter E•I* complex, and KI* represents the dissociation constant of the E•I* complex. The KI values were determined by initiating the enzymatic reactions with the LuxS enzyme (no preincubation), whereas the KI* values were obtained by incubating the enzyme with the inhibitors for 30 min at room temperature prior to initiating the reactions by the addition of SRH (KM = 2.4 µM) as the last component. The rate constant k could not be determined accurately, because the relatively fast E·I to E·I* conversion makes the KI measurement less reliable. Among the three compounds, SXH (16) was most potent, having KI and KI* values of 4.2 and 0.43 µM, respectively (Table 1). The 3-fluorinated 21a had KI and KI* values of 7.7 and 2.8 µM, respectively. The glycoside was least potent (KI = 42 µM and KI* = 8.8 µM), probably because the methyl group at the C1 position creates steric clashes with the active-site residues.
Figure 4.
Time-dependent inhibition of VhLuxS by compound 21a.
Co-VhLuxS (96 µM) was preincubated with the indicated concentrations of compound 21a (0.2–4.0 mM) at room temperature. At varying time points (0–2 h), 10-µL aliquots were withdrawn and rapidly diluted into a 1.0-mL solution containing 20 µM SRH and the rate of homocysteine formation was monitored with DTNB (absorbance change at 412 nm).
The observed slow-binding behavior of compounds 16, 21a, and 21b is very similar to that of [3-F]SRH (22) and [3-Br]SRH (23), both of which exhibited time-dependent inhibition of LuxS and their time dependency was caused by enzyme-catalyzed release of halide ions.35 Because compounds 16 and 21a are structurally similar to 22 and 23, we hypothesize that compounds 16 and 21a may undergo similar structural changes in the LuxS active site (e.g., the formation of 2-ketone intermediate-like species). The inverted stereochemistry at the C3 position in SXH would prevent the conversion of the intermediate into products. Unfortunately, all of our attempts to isolate/detect the altered inhibitor species (I*) failed. Thus, we cannot rule out the possibility that the observed slow-binding inhibition was caused by the conversion of E·I into E*·I, where E* is LuxS in an alternative conformation induced by inhibitor binding. The origin of time dependency of glycoside 21b is less clear, as it cannot undergo ring opening and therefore any catalyzed transformation.
Conclusion
In this work, we have synthesized several SRH analogues that lack an enolizable hydroxyl group at the carbon-3 position. Their evaluation against LuxS showed that removal or methylation of the C3-OH resulted in simple competitive inhibitors of moderate potency, while inversion of the C3 stereochemistry or substitution of fluorine for C3-OH resulted in slow-binding inhibition with the improved potency.
Experimental Section
1H (Me4Si) NMR spectra were determined at 400 or 600 MHz, 13C (Me4Si) at 100.6 MHz and 19F (CFCl3) at 376.5 MHz with solution in CDCl3 unless otherwise noted. Mass spectra (MS) were obtained by atmospheric pressure chemical ionization (APCI) and HRMS by electron impact techniques unless otherwise noted. Reagent grade chemicals were used and solvents were dried by reflux over and distillation from CaH2 under an argon atmosphere except THF (K/benzophenone). TLC was performed on Merck kieselgel 60-F254 precoated aluminium sheets, and products were detected by visualization with Ce(SO4)2/(NH4)6Mo7O24•4H2O/H2SO4/H2O reagent or with 254 nm light. Merck kieselgel 60 (230–400 mesh) was used for column chromatography. HPLC purifications were performed using XTerra® preparative RP18 OBD™ column (5 µm 19 × 150 mm) with program using CH3CN/H2O as a mobile phase.
3-Deoxy-1,2-O-isopropylidene-α-D-erythro-pentofuranose (1)
H5IO6 (922 mg, 4.1 mmol) was added to a stirred solution of diacetone 3-deoxyglucose30 (500 mg, 2.05 mmol) in dried EtOAc (40 mL) at ambient temperature. A precipitate appeared within the first five minutes and after 15 min, the somehow unstable 3-deoxy-1,2-O-isopropylidene-α-D-erythro-pentodialdo-1,4-furanose aldehyde can be detected on TLC and 1H NMR spectra (δ 9.75 (d, J5–4 = 4.8 Hz, ~0.9, H5). The white precipitate was filtered off and was washed with EtOAc (3 × 5 mL). The combined organic layer was evaporated, and the residue was quickly dissolved in EtOH (30 mL). Sodium borohydride (115 mg, 3.0 mmol) and water (10 mL) was added until all of the sodium borohydride was dissolved. After 45 min, the reaction mixture was evaporated, and the resulting residue was dissolved in EtOAc and washed with NaHCO3. The organic layer was dried (Na2SO4), evaporated and flash column chromatographed (40 → 60% EtOAc/hexane) to give 136 (217 mg, 61%).
3-Deoxy-l,2-O-isopropylidene-5-O-methylsulfonyl-α-D-erythro-pentofuranose (4)
Triethylamine (125 µL, 90.9 mg, 0.9 mmol) and CH3SO2Cl (35 µL, 51.3 mg, 0.45 mmol) were added to a stirred solution of 1 (52 mg, 0.3 mmol) in anhydrous CH2Cl2 (10 mL) under N2 at 0°C. After 10 min, the reaction mixture was partitioned (NaHCO3/H2O//CHCl3), and the organic layer was washed (brine), dried (Na2SO4) and evaporated to yield 437 (73 mg, 97%) of sufficient purity to be used in the next step: 1H NMR δ 1.34 (s, 3H), 1.53 (s, 3H), 1.80 (ddd, J = 4.7, 10.7, 13.5 Hz, 1H), 2.14 (dd, J = 4.2, 13.2 Hz, 1H), 3.08 (s, 3H), 4.27 (dd, J = 4.9, 11.4 Hz, 1H), 4.45 (dd, J = 2.8, 11.2 Hz, 1H), 4.44–4.53 (m, 1H), 4.79 (t, J = 4.2 Hz, 1H), 5.85 (d, J = 3.6 Hz, 1H).
l,2-O-Isopropylidene-3-O-methyl-5-O-methylsulfonyl-α-D-xylofuranose (5)
Treatment of 238 (50 mg, 0.25 mmol; prepared in 77% yield by dehomologation31 of diacetone 3-O-methylglucose39 as described for 1) with triethylamine (104 µL, 76 mg, 0.75 mmol) and CH3SO2Cl (30 µL, 43 mg, 0.38 mmol), as described for 4, gave 5 (68 mg, 96%) of sufficient purity to be used in the next step: 1H NMR δ 1.35 (s, 3H), 1.55 (s, 3H), 3.08 (s, 3H), 3.42 (s, 3H), 3.81 (d, J = 3.2 Hz, 1H), 4.37–4.51 (m, 3H), 4.62 (d, J = 3.8 Hz, 1H), 5.94 (d, J = 3.7 Hz, 1H); HRMS (ESI) m/z calcd for C10H19O7S [M+H]+ 283.0851, found 283.0844.
l,2-O-Isopropylidene-3-O-methyl-5-O-p-toluenesulfonyl-α-D-ribofuranose (6)
TsCl (93 mg, 0.49 mmol) was added to a stirred solution of 340 (90 mg, 0.45 mmol; prepared in 67% yield by dehomologation31 of diacetone 3-O-methylallose40 as described for 1) in pyridine (1 mL) at 0°C. After 12 h, an additional TsCl (93 mg, 0.49 mmol) was added and the stirring was continued for 8 h. Pyridine was evaporated/coevaporated with toluene (3×), and the residue was dissolved in EtOAC and washed with NaHCO3 and NaCl. The organic layer was evaporated and column chromatographed (hexane/EtOAc, 1:1) to give 640 (117 mg, 72%) of sufficient purity to be used in the next step: 1H NMR δ 1.34 (s, 3H), 1.52 (s, 3H), 2.44 (s, 3H), 3.44 (s, 3H), 3.64 (dd, J = 4.2, 9.0 Hz, 1H), 4.07 (dt, J = 2.4, 9.1 Hz, 1H), 4.18 (dd, J = 3.3, 11.2 Hz, 1H), 4.27 (dd, J = 2.1, 11.2 Hz, 1H), 4.64 (t, J = 3.9 Hz, 1H), 5.66 (d, J = 3.6 Hz, 1H), 7.34 (d, J = 8.4 Hz, 2H), 7.79 (d, J = 8.4 Hz, 2H).
S-(3,5-Dideoxy-l,2-O-isopropylidene-α-D-erythro-pentofuranos-5-yl)homocysteine (7)
Compound 4 (45.6 mg, 0.18 mmol) was added to 1 M NaOH/H2O (5 mL) and degassed with N2 for 30 min. D/L-Homocysteine (36.5 mg, 0.27 mmol) was then added, and the suspension was heated to 60°C. After 12 h, the reaction mixture was cooled to room temperature, neutralized with dilute HCl to pH ~7 and washed with EtOAc (3×). The water layer was evaporated and purified by HPLC (25% CH3CN/H2O for 35 min at 2.5 mL/min; tR = 12.6 min) to give 7 (16 mg, 31%): 1H NMR (D2O) δ 1.39 (s, 3H), 1.55 (s, 3H), 1.83 (ddd, J = 4.7, 10.9, 14.0 Hz, 1H), 2.08–2.21 (m, 2H), 2.24 (dd, J = 4.3, 14.0, 1H), 2.72–2.78 (m, 2H), 2.81 (dd, J = 6.8, 13.9 Hz, 1H), 2.93 (dd, J = 4.5, 13.8 Hz, 1H), 3.88 (t, J = 6.3 Hz, 1H), 4.46 (dddd, J = 4.3, 4.5, 6.8, 10.9 Hz, 1H), 4.98 (t, J = 4.1 Hz, 1H), 5.94 (d, J = 3.7 Hz, 1H); MS m/z 292 (100%, MH+).
S-(5-Deoxy-l,2-O-isopropylidene-3-O-methyl-α-D-xylofuranos-5-yl)homocysteine (8)
Treatment of 5 (35 mg, 0.12 mmol) with D/L-homocysteine (25 mg, 0.19 mmol), as described for 7, and purification by HPLC (5% CH3CN/H2O for 45 min at 2.5 mL/min; tR = 30 min) gave 8 (11 mg, 28%): 1H NMR (D2O) δ 1.36 (s, 3H), 1.53 (s, 3H), 1.90–2.10 (m, 2H), 2.65–2.73 (m, 2H), 2.82 (dd, J = 7.5, 13.6 Hz, 1H), 2.87 (dd, J = 6.6, 13.6 Hz, 1H), 3.46 (s, 3H), 3.57 ("t", J = 6.9 Hz, 1H), 3.91 (d, J = 2.9 Hz, 1H), 4.38 (dt, J = 2.9, 7.2 Hz, 1H), 4.88 (d, J = 3.9 Hz, 1H), 5.98 (d, J = 3.9 Hz, 1H); 13C NMR (D2O) δ 24.9, 25.4, 27.9, 28.0, 28.42, 32.71, 54.54, 57.42, 79.8, 79.9, 81.0, 83.3, 104.3, 113.8, 174.0; HRMS (AP-ESI) m/z calcd for C13H24NO6S [M+H]+ 322.1325, found 322.1321.
S-(5-Deoxy-l,2-O-isopropylidene-3-O-methyl-α-D-ribofuranos-5-yl)homocysteine (9)
Treatment of 6 (48 mg, 0.14 mmol) with D/L-homocysteine (28 mg, 0.06 mmol), as described for 7, and purification by HPLC (5% CH3CN/H2O for 45 min at 2.5 mL/min; tR = 35.0 min) gave 9 (13 mg, 30%): 1H NMR (D2O) δ 1.40 (s, 3H), 1.56 (s, 3H), 2.03–2.23 (m, 2H), 2.72 (t, J = 7.6 Hz, 2H), 2.79 (dd, J = 7.1, 14.3 Hz, 1H), 3.03 (dd, J = 3.2, 14.3 Hz, 1H), 3.48 (s, 3H), 3.80 (dd, J = 4.1, 9.0 Hz, 1H), 3.78–3.83 (m, 1H), 4.12 (ddd, J = 3.1, 7.1, 9.3 Hz, 1H), 4.97 (t, J = 3.9 Hz, 1H), 5.90 (d, J = 3.7, 1H); MS m/z 322 (100%, MH+).
S-(3,5-Dideoxy-D-erythro-pentofuranos-5-yl)homocysteine (10)
A solution of 7 (9 mg, 0.03 mmol) in TFA/H2O (9:1, 3 mL) was stirred for 45 min. at 0°C (ice-bath). The reaction mixture was evaporated and coevaporated [toluene (3×), CH3CN (3×)] and the residue was purified by HPLC (5% CH3CN/H2O for 25 min at 2.5 mL/min) to yield 7 (7 mg, 90%, α/β, ~1:3; tR = 12.6 min): 1H NMR (D2O) δ 2.02 (ddd, J = 4.7, 9.4, 14.0 Hz, 1H), 2.06–2.25 (m, 3H), 2.69–2.78 (m, 2H), 2.78–2.91 (m, 2H), 3.87 ("t", J = 6.1 Hz, 1H), 4.26 (d, J = 4.6 Hz, 0.75H), 4.35 ("dt", J = 4.1, 6.8 Hz, 0.25H), 4.44–4.53 (m, 1H), 5.26 (s, 0.75H), 5.35 (d, J = 4.0 Hz, 0.25H); HRMS (LCT-ESI) m/z calcd for C9H17O5NSNa [M+Na]+ 274.0725, found 274.0725.
S-(5-Deoxy-3-O-methyl-D-xylofuranos-5-yl)homocysteine (11)
Treatment of 8 (22 mg, 0.07 mmol) with TFA/H2O, as described for 10, and purification by HPLC (5% CH3CN/H2O for 45 min at 2.5 mL/min; tR = 20 min) gave 11 (12 mg, 60%; α/β, ~1:1): 1H NMR (D2O) δ 2.06–2.24 (m, 2H), 2.68–2.76 (m, 2H), 2.78–2.85 (m, 1.5H), 2.91 (dd, J = 5.9, 13.7 Hz, 0.5H), 3.41 (s, 1.5H), 3.44 (s, 1.5H), 3.80 (dd, J = 1.7, 4.7 Hz, 0.5H), 3.83–3.89 (m, 1H), 3.92 (dd, J = 4.0, 4.8 Hz, 0.5H), 4.20 (s, 0.5H), 4.24 (t, J = 4.1 Hz, 0.5H), 4.38–4.47 (m, 1H), 5.20 (s, 0.5H), 5.39 (d, J = 4.4 Hz, 0.5H); HRMS (LCT-ESI) m/z calcd for C10H19O6NSNa [M+Na]+ 304.0831, found 304.0829.
S-(5-Deoxy-3-O-methyl-D-ribofuranos-5-yl)homocysteine (12)
Treatment of 9 (10 mg, 0.03 mmol) with TFA/H2O, as described for 10, and purification by HPLC (5% CH3CN/H2O for 45 min at 2.5 mL/min; tR = 20 min) gave 12 (5 mg, 59%; α/β, ~3:7): 1H NMR (D2O) δ 2.07–2.26 (m, 2H), 2.70–2.79 (m, 2H), 2.79–2.99 (m, 2H), 3.44 (s, 0.9H), 3.47 (s, 2.1H), 3.78 (t, J = 5.0 Hz, 0.3H), 3.84 (t, J = 5.5 Hz, 0.3H), 3.86 (dd, J = 5.5, 7.0 Hz, 0.7H), 3.94 (dd, J = 4.5, 6.5 Hz, 0.7H), 4.07–4.14 (m, 1H), 4.23 (dd, J = 1.3, 4.3 Hz, 0.7H), 4.29 ("t" J = 4.6 Hz, 0.3H) 5.26 (s, 0.7H), 5.37 (d, J = 4.0 Hz, 0.3H); HRMS (LCT-ESI) m/z calcd for C10H19O6NSNa [M+Na]+ 304.0831, found 304.0827.
1,2-O-Isopropylidene-3,5-O-sulfonyl-α-D-xylofuranose (14)
To a stirred solution of diol 1333 (0.4 g, 2.11 mmol) in CH2Cl2 (12 mL) were added TEA (1.2 mL, 8.42 mmol) and SOCl2 (2 M/DCM; 2.1 mL, 4.22 mmol) at −78 °C under argon atmosphere. The mixture was allowed to warm to 0 °C and stirred until the reaction was completed (1 h). The reaction mixture was diluted in DCM (50 mL), washed with H2O (20 mL), dried over MgSO4, and concentrated to dryness. The residue was dissolved in CCl4 (6 mL) and CH3CN (6 mL). To the resulting solution was added H2O (9 mL) followed by RuCl3 hydrate (15 mg) and NaIO4 (0.9 g, 4.22 mmol) at 0 °C. The mixture was stirred for 2 h, diluted in EtOAc (50 mL), washed with H2O (20 mL), dried over MgSO4, and concentrated in reduced pressure. The crude product was purified by silica gel chromatography (EtOAc/hexane, 1:2) to afford the cyclic sulfate 14 (0.39 g, 74%) as a white solid: 1H NMR δ 1.33 (s, 3H), 1.49 (s, 3H), 4.24 (m, 1H), 4.70 (d, J = 3.6 Hz, 1H), 4.81 (d, J = 12.8 Hz, 1H), 4.95 (dd, J = 2.0, 12.8 Hz, 1H), 5.16 (d, J = 2.0 Hz, 1H), 6.03 (d, J = 4.0 Hz, 1H); 13C NMR δ 26.1, 26.5, 69.3, 72.4, 82.8, 86.5, 104.7, 113.1; HRMS (ESI) m/z calcd for C8H12O7SNa+ [M+Na+] 275.0201, found 275.0212.
N-tert-Butoxycarbonyl-S-(5-deoxy-1,2-O-isopropylidene-α-D-xylofuranos-5-yl)-L-homocysteine tert-Butyl ester (15)
To a solution of BocNH-CH(CH2CH2SH)-CO2tBu (1.1 g, 3.77 mmol) in DMF (15 mL) was added BuLi (2.5 M/hexane; 0.9 mL, 2.26 mmol) at 0 °C under argon atmosphere. After stirring for 5 min, a solution of cyclic sulfate 14 (0.38 g, 1.51 mmol) dissolved in DMF (10 mL) was added. The mixture was stirred for 4 h, diluted with EtOAc (75 mL), washed with H2O (25 mL) and brine (25 mL). The organic layer was dried over MgSO4 and concentrated to dryness. The residue was dissolved in THF (3 mL) and concentrated H2SO4 (2 µL) and H2O (2 µL) were added at 0 °C. The mixture was stirred for 1 h at 0 °C and then partitioned between EtOAc (100 mL) and 5% NaHCO3 solution (40 mL). The organic layer was collected, washed with H2O (20 mL) and brine (20 mL), dried over MgSO4 and concentrated under vacuum. The crude product was purified by silica gel chromatography (EtOAc/hexane, 2:3) to afford alcohol 15 (0.47 g, 67%) as a white solid: 1H NMR δ 1.29 (s, 3H), 1.43 (s, 9H), 1.45 (s, 9H), 1.48 (s, 3H), 1.87 (m, 1H), 2.09 (m, 1H), 2.50 (d, J = 5.6 Hz, 1H), 2.56–2.69 (m, 2H), 2.81 (dd, J = 8.4, 13.2 Hz, 1H), 2.87 (dd, J = 5.2, 13.2 Hz, 1H), 4.21–4.30 (m, 3H), 4.51 (d, J = 3.6 Hz, 1H), 5.10 (d, J = 5.6 Hz, 1H), 5.90 (d, J = 3.6 Hz, 1H); 13C NMR δ 26.2, 26.7, 28.0, 28.3, 28.6, 29.2, 33.0, 53.2, 74.9, 79.7, 80.0, 82.4, 85.2, 104.7, 111.6, 155.5, 171.4; HRMS (ESI) m/z calcd for C21H37NO8SNa+ [M+Na+] 486.2138, found 486.2141.
S-(5-Deoxy-D-xylofuranos-5-yl)-L-homocysteine (16)
Alcohol 15 (40 mg, 0.09 mmol) was treated with TFA containing 10% anisole and 10% water (5 mL) at room temperature for 6 h. The volatile solvents were removed by evaporation, and the residue was dissolved in H2O (3 mL) and washed with CH2Cl2 (3 × 2 mL). The aqueous solution was lyophilized to give the desired SXH 16 (20 mg, 61%) as a trifluoroacetate salt: 1H NMR (250 MHz, D2O) δ 1.85–2.10 (m, 2H), 2.35–2.70 (m, 4H), 3.60–4.40 (m, 3H), 4.96 (s, 1H), 5.20 (d, J = 4.3 Hz, 1H); HRMS (ESI) m/z calcd for C9H17NO6SNa+ [M+Na+] 290.0674, found 290.0684.
3-Deoxy-3-fluoro-l,2-O-isopropylidene-α-D-xylofuranose (18)
Treatment of 1734 (75 mg, 0.29 mmol) with H5IO6 (77 mg, 0.34 mmol) and NaBH4 (16.5 mg, 0.43 mmol), as described for 1, followed by flash column chromatography (EtOAc/hexane, 1:1) gave 18 (39 mg, 71%) with data as reported:41 19F NMR δ −208.66 (ddd, J = 11.2, 30.1, 50.4 Hz).
3-Deoxy-3-fluoro-l,2-O-isopropylidene-5-O-p-toluenesulfonyl-α-D-xylofuranose (19a)
Treatment of 18 (66 mg, 0.34 mmol) with TsCl (130 mg, 0.68 mmol) in pyridine (1 mL), as described for 6, followed by flash column chromatography (EtOAc/hexane, 25:75) gave 19a42 (72 mg, 62%): 1H NMR δ 1.32 (s, 3H), 1.48 (s, 3H), 2.47 (s, 3H), 4.17–4.28 (m, 2H), 4.41 (dtd, J = 2.2, 6.3, 28.2 Hz, 1H), 4.67 (dd, J = 3.8, 10.6 Hz, 1H), 4.96 (dd, J = 2.2, 50.1 Hz, 1H), 5.93 (d, J = 3.6 Hz, 1H), 7.38 (d, J = 8.3 Hz, 2H), 7.83 (d, J = 8.3 Hz, 2H); 19F NMR δ −208.85 (ddd, J = 10.1, 27.7, 49.7 Hz).
3-Deoxy-3-fluoro-1,2-O-isopropylidene-5-O-methanesulfonyl-α-D-xylofuranose (19b)
Et3N (0.115 mL, 84 mg, 0.83 mmol) and MsCl (0.032 mL, 47 mg, 0.41 mmol) were added dropwise to a stirred solution of 18 (53 mg, 0.27 mmol) in anhydrous CH2Cl2 (5 mL) at ambient temperature. After 15 min, the reaction mixture was quenched with saturated NaHCO3/H2O, and extracted with CHCl3. The combined organic layer was washed with brine, dried (Na2SO4) and was evaporated and column chromatographed (20 → 40% EtOAc/hexane) to give 19b (54 mg, 75%) as a white solid: 1H NMR δ 1.36 (s, 3H), 1.52 (s, 3H), 3.10 (s, 3H), 4.42–4.48 (m, 2H), 4.52 (dddd, J = 2.3, 5.5, 7.1, 28.5 Hz, 1H), 4.74 (dd, J = 3.7, 10.8 Hz, 1H), 5.04 (dd, J = 1.8, 50.0 Hz, 1H), 6.03 (d, J = 3.7 Hz, 1H); 13C NMR δ 26.2, 26.7, 37.6, 65.9 (d, J = 11.0 Hz) 77.4 (d, J = 18.7 Hz), 82.4 (d, J = 31.7 Hz), 93.7 (d, J = 184.8 Hz), 105.1, 112.8; 19F NMR δ −208.7 (ddd, J = 10.6, 27.7, 50.2 Hz); MS m/z 271 (MH+).
S-(3,5-Dideoxy-3-fluoro-l,2-O-isopropylidene-α-D-xylofuranos-5-yl)homocysteine [20a(9R/S)]
A solution of 19a (18.5 mg, 0.05 mmol) in 2 M NaOH/H2O-EtOH solution (4 mL, 1:1) was degassed with N2 for 30 min. D/L-Homocysteine (11 mg, 0.08 mmol) was added, and the solution was heated to 60 °C. After 36 h, the reaction mixture was cooled to room temperature, neutralized with conc. HCl and washed with EtOAc (3×). The water layer was evaporated and purified by HPLC (5% CH3CN/H2O for 60 min at 2.5 mL/min; tR = 30 min) to give 20a (5 mg, 32%; 9R/S, ~1:1): 1H NMR (D2O) δ 1.37 (s, 3H), 1.52 (s, 3H), 2.05–2.26 (m, 2H), 2.72–2.78 (m, 2H), 2.86–2.97 (m, 2H), 3.83 (dd, J = 5.7, 7.0 Hz, 1H), 4.45 (dtd, J = 2.0, 6.6, 28.7 Hz, 1H), 4.94 (dd, J = 3.9, 10.8 Hz, 1H), 5.12 (dd, J = 2.0, 49.1 Hz, 1H), 6.10 (d, J = 4.0 Hz, 1H); 19F NMR (D2O) δ −208.45 (ddd, J = 10.7, 28.9, 49.1 Hz, 0.5F; 20a-9S), −208.43 (ddd, J = 10.7, 28.9, 49.1 Hz, 0.5F; 20a-9R); MS (APCI) m/z 310 (MH+).
N-(tert-Butoxycarbonyl)-S-(3,5-dideoxy-3-fluoro-1,2-O-isopropylidene-α-D-xylofuranos-5-yl)-L-homocysteine tert-Butyl ester (20b)
Step a. H2O (0.24 mL) and tris(2-carboxyethyl)phosphine hydrochloride (88 mg, 0.31 mmol) were added to a stirred solution of N,N'-di(tert-butoxycarbonyl)-L-homocystine di(tert-butyl) ester (160 mg, 0.28 mmol) in anhydrous DMF (2.4 mL) at ambient temperature under Ar atmosphere. After 32 h, the reaction mixture [TLC (EtOAc/hexane, 2:8) showed conversion of disulfide (Rf 0.55) into thiol (Rf 0.65)] was partitioned between EtOAc, and saturated NaHCO3/H2O. After separation, the aqueous layer was extracted with EtOAc, and the combined organic layer was washed with brine, dried (Na2SO4), and concentrated to give N-tert-butoxycarbonyl-L-homocysteine tert-butyl ester22 (159 mg,99%) as colorless oil of sufficient purity to be directly used in next step. Step b. A freshly prepared homocysteine (80 mg, 0.275 mmol) was dissolved in anhydrous DMF (0.5 mL), and was stirred under a vigorous stream of argon for 10 min at 0°C (ice-bath). Next, a solution of LDA (138 µL, 2.0 M/THF-heptane) was added dropwise and after an additional 10 min 19b (31 mg, 0.115 mmol) in anhydrous DMF (0.5 mL) was added via syringe. After 15 min, ice-bath was removed and the reaction mixture was stirred for 28 h at ambient temperature (TLC showed consumption of 19b). Ice-cold saturated NH4Cl/H2O was added and the resulting suspension was diluted with EtOAc. The organic layer was separated and the aqueous layer was extracted with EtOAc. The combined organic layer was washed with brine, dried (Na2SO4) and was evaporated to give 94 mg of yellowish oily residue. This crude product was column chromatographed on silica gel (hexane/EtOAc, 4:1) to give 20b as an colorless oil (49 mg, 92%): 1H NMR δ 1.33 (s, 3H), 1.45 (s, 9H), 1.48 (s, 9H), 1.51 (s, 3H), 1.84–1.95 (m, 1H), 2.05–2.16 (m, 1H), 2.63 (t, J = 7.7 Hz, 2H), 2.78 (dd, J = 8.4, 13.7 Hz, 1H), 2.86 (ddd, J = 1.4, 6.2, 13.7 Hz, 1H), 4.23–4.32 (m, 1H), 4.31 (dm, J = 28.2 Hz, 1H), 4.70 (dd, J = 3.8, 10.7 Hz, 1H), 4.98 (dd, J = 1.9, 49.9 Hz, 1H), 5.12 (br. d, J = 6.7 Hz, 1H), 5.96 (d, J = 3.9 Hz, 1H); 13C NMR δ 26.2, 26.7, 28.0, 28.3, 28.6, 28.7, 33.2, 53.3, 79.8, 80.1 (d, J = 19.4 Hz), 82.2, 82.41 (d, J = 32.7 Hz), 93.78 (d, J = 185.0 Hz), 104.8, 112.2, 155.3, 171.3; 19F NMR δ −208.16 (ddd, J = 10.4, 28.4, 49.8 Hz); HRMS (AP-ESI) m/z calcd for C21H37FNO7S [M+H] + 466.2269; found 466.2265.
S-(3,5-Dideoxy-3-fluoro-D-xylofuranos-5-yl)homocysteine [21a(9R/S)]
Treatment of 20a (6 mg, 0.02 mmol; 9R/S, ~1:1) with TFA/H2O (9:1, 5 mL), as described for 10, and purification by HPLC (preparative RP-C18 column, 5% CH3CN/H2O for 45 min at 2.5 mL/min; tR = 16.5 min) gave 21a (3 mg, 55%; 9R/S, ~1:1, α/β, ~2:3): 19F NMR (D2O) δ −202.41 (ddd, J = 18.7, 25.4, 52.1 Hz, 0.2F; α, 21a-9S), −202.38 (ddd, J = 18.7, 25.4, 52.1 Hz, 0.2F; α, 21a-9R), −200.55 (ddd, J = 12.6, 28.0, 50.6 Hz, 0.3F; β, 21a-9S), −200.52 (ddd, J = 12.6, 28.0, 50.6 Hz, 0.3F; β, 21a-9R); 1H NMR spectra as reported below for 21a-9S; MS (APCI) m/z 270 (MH+); HRMS (LCT-ESI) m/z calcd for C9H16FNO5S [M+H]+ 270.0811; found 270.0798.
S-(3,5-Dideoxy-3-fluoro-D-xylofuranos-5-yl)-L-homocysteine (21a)
Compound 20b (17 mg, 0.037 mmol) was dissolved in TFA (1 mL) and the resulting mixture was stirred at ambient temperature for 4 h and was evaporated and coevaporated with toluene. The crude mixture [19F NMR (D2O) δ −208.45 (0.82F, 20a-9S), −202.41 (0.07F; α, 21a-9S), −200.55 (0.11F; β, 21a-9S)] was treated (2 h) with TFA/H2O (9:1, 2 mL), as described for 10, and purified on HPLC (preparative RP-C18 column, 5% CH3CN/H2O for 45 min at 2.5 mL/min; tR = 16.5 min) to give 21a (4.7 mg, 46% overall; α/β, ~2:3): 1H NMR (D2O) δ 2.10–2.35 (m, 2H), 2.72–2.82 (m, 2H), 2.82–3.01 (m, 2H), 4.01 ("t", J = 6.1 Hz, 1H), 4.33 (d, J = 12.6 Hz, 0.6H), 4.40 (ddd, J = 2.4, 4.3, 8.6 Hz, 0.4H), 4.39–4.55 (m, 1H), 5.03 (dd, J = 3.3, 50.6 Hz, 0.6H), 5.10 (ddd, J = 2.5, 3.6, 52.1 Hz, 0.4H), 5.30 (s, 0.6H), 5.53 (dd, J = 1.0, 4.3 Hz, 0.4H); 19F NMR (D2O) δ −202.41 (ddd, J = 18.7, 25.4, 52.1 Hz, 0.4F; α), −200.55 (ddd, J = 12.6, 28.0, 50.6 Hz, 0.6F; β); MS (APCI) m/z 270 (MH+). HRMS (TOF MS-ESI) m/z calcd for C9H16FNNaO5S [M+Na]+ 292.0631; found 292.0611.
S-(3,5-Dideoxy-3-fluoro-1-O-methyl-D-xylofuranos-5-yl)-L-homocysteine (21b)
BCl3 solution (1.0 M/CH2Cl2; 0.2 mL) was added dropwise to a stirred solution of 20b (25 mg, 0.054 mmol) in CH2Cl2 (3 mL) at −50 °C, under Ar atmosphere. The reaction mixture was stirred at same temperature for 20 min, and reaction was quenched with MeOH (2 mL). The volatiles were evaporated and resulting crude product was purified on RP-HPLC (as described for 21a) to give 21a (1 mg, 7%; α:β 1:3; tR = 16.5 min), and 21b (4.5 mg, 29%; α:β 45:55; tR = 24.5 min). Compound 21b had: 1H NMR (D2O) δ 2.07–2.28 (m, 2H), 2.76 (q, J = 7.1 Hz, 2H), 2.80–3.00 (m, 2H), 3.43 (s, 0.55H), 3.46 (s, 0.45H), 3.89 ("t", J = 6.1 Hz, 1H), 4.30–4.75 (m, 1H), 4.38 (d, J = 12.2 Hz, 0.55H), 4.47 (ddd, J = 2.9, 4.5, 23.2 Hz, 0.45H), 4.97 (s, 0.55H), 5.05 (dd, J = 4.0, 51.2 Hz, 0.55H), 5.09 (ddd, J = 3.0, 4.6, 53.5 Hz, 0.45H), 5.12 (d, J = 4.6 Hz, 0.45H); 13C NMR (D2O) δ 27.3, 27.4, 29.2, 29.3, 30.2, 30.2, 53.6, 55.4, 55.6, 75.6 (d, J = 27.1 Hz), 76.9 (d, J = 19.7 Hz), 77.3 (d, J = 27.1 Hz), 81.2 (d, J = 19.5 Hz), 94.8 (d, J = 185.2 Hz), 96.9 (d, J = 184.2 Hz), 102.1 (d, J = 6.0 Hz), 108.7, 173.9; 19F NMR (D2O) δ −201.21 (dt, J = 22.8, 53.6 Hz, 0.45F; α), −199.51 (ddd, J = 11.9, 27.1, 50.8 Hz, 0.55F; β); MS (AP-CI) m/z 284 (MH)+. HRMS (TOF MS-ESI) m/z calcd for C10H18FNNaO5S [M+Na]+ 306.0787; found 306.0775
LuxS assay
Inhibition assays were performed in a buffer containing 50 mM HEPES (pH 7.0), 150 mM NaCl, 150 µM 5,5'-dithio-bis-(2-nitrobenzoic acid) (DTNB) and various concentrations of SRH (0–55 µM) and inhibitors (0–1 mM). The reactions were initiated by the addition of Co-BsLuxS (final concentration 0.4–0.5 µM) and monitored continuously at 412 nm (ε = 14150 M−1 cm−1) in a Perkin-Elmer λ25 UV-VIS spectrophotometer at room temperature (23 °C). The initial rates recorded from the early regions of the progress curves were fitted into the Lineweaver-Burk equation 1/V = KM’/(kcat [E]0) × 1/[S] + 1/(kcat [E]0) and the Michaelis-Menten equation V = kcat [E]0 [S]/(KM’ + [S]) using KaleidaGraph 3.5 to determine the inhibition pattern. KI values were calculated from the equation KM’ = KM × (1 + [I]/KI), where KM = 2.2 µM.
Acknowledgements
This work was supported by grants from the NIH (SC1CA138176 and AI062901). J.R was sponsored by MBRS RISE program (NIH/NIGMS; R25GM61347).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee JE, Cornell KA, Riscoe MK, Howell PL. Structure. 2001;9:941–953. doi: 10.1016/s0969-2126(01)00656-6. [DOI] [PubMed] [Google Scholar]
- 2.Singh V, Shi W, Almo SC, Evans GB, Furneaux RH, Tyler PC, Painter GF, Lenz DH, Mee S, Zheng R, Schramm VL. Biochemistry. 2006;45:12929–12941. doi: 10.1021/bi061184i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pei D, Zhu J. Curr. Opin. Chem. Biol. 2004;8:492–497. doi: 10.1016/j.cbpa.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Schauder S, Shokat K, Surette MG, Bassler BL. Mol. Microbiol. 2001;41:463–476. doi: 10.1046/j.1365-2958.2001.02532.x. [DOI] [PubMed] [Google Scholar]
- 5.Winzer K, Hardie KR, Burgess N, Doherty N, Kirke D, Holden MTG, Linforth R, Cornell KA, Taylor AJ, Hill PJ, Williams P. Microbiology. 2002;148:909–922. doi: 10.1099/00221287-148-4-909. [DOI] [PubMed] [Google Scholar]
- 6.Miller ST, Xavier KB, Campagna SR, Taga ME, Semmelhack MF, Bassler BL, Hughson FM. Mol. Cell. 2004;15:677–687. doi: 10.1016/j.molcel.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, Bassler BL, Hughson FM. Nature. 2002;415:545–549. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 8.Meijler MM, Hom LG, Kaufmann GF, McKenzie KM, Sun C, Moss JA, Matsushita M, Janda KD. Angew. Chem. Int. Ed. 2004;43:2106–2108. doi: 10.1002/anie.200353150. [DOI] [PubMed] [Google Scholar]
- 9.Lowery CA, Park J, Kaufmann GF, Janda KD. J. Am. Chem. Soc. 2008;130:9200–9201. doi: 10.1021/ja802353j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semmelhack MF, Campagna SR, Federle MJ, Bassler BL. Org. Lett. 2005;7:569–572. doi: 10.1021/ol047695j. [DOI] [PubMed] [Google Scholar]
- 11.Semmelhack MF, Campagna SR, Hwa C, Federle MJ, Bassler BL. Org. Lett. 2004;6:2635–2637. doi: 10.1021/ol048976u. [DOI] [PubMed] [Google Scholar]
- 12.De Keersmaecker SCJ, Varszegi C, van Boxel N, Habel LW, Metzger K, Daniels R, Marchal K, De Vos D, Vanderleyden J. J. Biol. Chem. 2005;280:19563–19568. doi: 10.1074/jbc.M412660200. [DOI] [PubMed] [Google Scholar]
- 13.Frezza M, Soulere L, Queneau Y, Doutheau A. Tetrahedron Lett. 2005;46:6495–6498. [Google Scholar]
- 14.Frezza M, Balestrino D, Soulère L, Reverchon S, Queneau Y, Forestier C, Doutheau A. Eur. J. Org. Chem. 2006:4731–4736. [Google Scholar]
- 15.Frezza M, Soulere L, Balestrino D, Gohar M, Deshayes C, Queneau Y, Forestier C, Doutheau A. Bioorg. Med. Chem. Lett. 2007;17:1428–1431. doi: 10.1016/j.bmcl.2006.11.076. [DOI] [PubMed] [Google Scholar]
- 16.Waters CM, Bassler BL. Annu. Rev. Cell Dev. Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 17.Bassler BL, Losick R. Cell. 2006;125:237–246. doi: 10.1016/j.cell.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Camilli A, Bassler BL. Science. 2006;311:1113–1116. doi: 10.1126/science.1121357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lebeer S, De Keersmaecker SCJ, Verhoeven TLA, Fadda AA, Marchal K, Vanderleyden J. J. Bacteriol. 2007;189:860–871. doi: 10.1128/JB.01394-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu J, Dizin E, Hu X, Wavreille A-S, Park J, Pei D. Biochemistry. 2003;42:4717–4726. doi: 10.1021/bi034289j. [DOI] [PubMed] [Google Scholar]
- 21.Rajan R, Zhu J, Hu X, Pei D, Bell CE. Biochemistry. 2005;44:3745–3753. doi: 10.1021/bi0477384. [DOI] [PubMed] [Google Scholar]
- 22.Zhu J, Hu X, Dizin E, Pei D. J. Am. Chem. Soc. 2003;125:13379–13381. doi: 10.1021/ja0369663. [DOI] [PubMed] [Google Scholar]
- 23.Zhu J, Patel R, Pei D. Biochemistry. 2004;43:10166–10172. doi: 10.1021/bi0491088. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J, Knottenbelt S, Kirk ML, Pei D. Biochemistry. 2006;45:12195–12203. doi: 10.1021/bi061434v. [DOI] [PubMed] [Google Scholar]
- 25.Turner MA, Yang X, Yin D, Kuczera K, Borchardt RT, Howell PL. Cell Biochem. Biophys. 2000;33:101–125. doi: 10.1385/CBB:33:2:101. [DOI] [PubMed] [Google Scholar]
- 26.Wnuk SF. Mini-Rev. Med. Chem. 2001;1:307–316. doi: 10.2174/1389557013406918. [DOI] [PubMed] [Google Scholar]
- 27.Alfaro JF, Zhang T, Wynn DP, Karschner EL, Zhou ZS. Org. Lett. 2004;6:3043–3046. doi: 10.1021/ol049182i. [DOI] [PubMed] [Google Scholar]
- 28.Shen G, Rajan R, Zhu J, Bell CE, Pei D. J. Med. Chem. 2006;49:3003–3011. doi: 10.1021/jm060047g. [DOI] [PubMed] [Google Scholar]
- 29.Wnuk SF, Lalama J, Garmendia CA, Robert J, Zhu J, Pei D. Bioorg. Med. Chem. 2008;16:5090–5102. doi: 10.1016/j.bmc.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robins MJ, Wilson JS, Hansske F. J. Am. Chem. Soc. 1983;105:4059–4065. [Google Scholar]
- 31.Xie M, Berges DA, Robins MJ. J. Org. Chem. 1996;61:5178–5179. [Google Scholar]
- 32.Miles RW, Nielsen LPC, Ewing GJ, Yin D, Borchardt RT, Robins MJ. J. Org. Chem. 2002;67:8258–8260. doi: 10.1021/jo020478g. [DOI] [PubMed] [Google Scholar]
- 33.Moravcová J, Capková J, Stanek J. Carbohydr. Res. 1994;263:61–66. [Google Scholar]
- 34.Tewson TJ, Welch MJ. J. Org. Chem. 1978;43:1090–1092. [Google Scholar]
- 35.Gopishetty B, Zhu J, Rajan R, Sobczak AJ, Wnuk SF, Bell CE, Pei D. J. Am. Chem. Soc. 2009;131:1243–1250. doi: 10.1021/ja808206w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiozaki M, Deguchi N, Mochizuki T, Wakabayashi T, Ishikawa T, Haruyama H, Kawai Y, Nishijima M. Tetrahedron. 1998;54:11861–11876. [Google Scholar]
- 37.Bentley N, Dowdeswell CS, Singh G. Heterocycles. 1995;41:2499–2506. [Google Scholar]
- 38.Yadav JS, Subba Reddy BV. Carbohydr. Res. 2000;329:885–888. doi: 10.1016/s0008-6215(00)00241-x. [DOI] [PubMed] [Google Scholar]
- 39.Yan S, Klemm D. Tetrahedron. 2002;58:10065–10071. [Google Scholar]
- 40.Haga M, Takano M, Tejima S. Carbohydr. Res. 1972;21:440–446. [Google Scholar]
- 41.Jeong LS, Lim BB, Marquez VE. Carbohydr. Res. 1994;262:103–114. doi: 10.1016/0008-6215(94)84007-5. [DOI] [PubMed] [Google Scholar]
- 42.Tsoukala E, Agelis G, Dolinsek J, Botic T, Cencic A, Komiotis D. Bioorg. Med. Chem. 2007;15:3241–3247. doi: 10.1016/j.bmc.2007.02.031. [DOI] [PubMed] [Google Scholar]