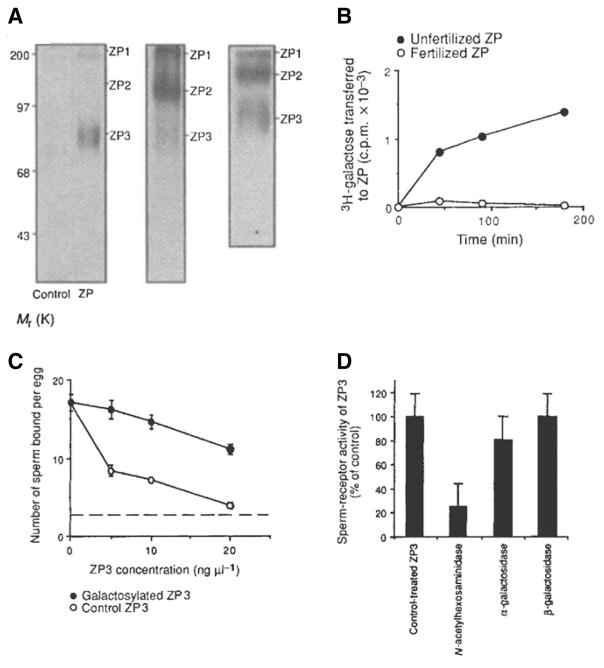
Fig. 1. Sperm galactosyltransferase (GalT) selectively recognizes glycoside substrates on ZP3 from fertilized eggs, although non-sperm GalT binds all ZP glycoproteins.
(A) (Left panel) Intact sperm and UDP-3[H]Gal were incubated with (ZP) or without (control) heat-solubilized ZP glycoproteins and the reaction products analyzed by SDS-PAGE and fluorography. Sperm GalT selectively binds and 3[H]galactosylates ZP3 glycans, but not ZP1 or ZP2. (Middle panel) The absence of 3[H] labeling of ZP1 and ZP2 is not the result of proteolysis during the incubation, as judged by parallel assays containing 125I-ZP glycoproteins incubated with sperm and unlabeled UDPGal. All three ZP glycoproteins remain intact during the incubation. (Right panel) Substituting sperm GalT with affinity-purified milk GalT results in 3[H]galactosylation of all three ZP glycoproteins, illustrating the strict substrate specificity of sperm GalT towards ZP3 substrates. (B) Kinetics of sperm GalT 3[H]galactosylation of ZP glycoproteins isolated from unfertilized and fertilized oocytes. In contrast to the presence of GalT substrates in unfertilized ZP, ZP collected from fertilized eggs no longer possess GalT substrates. (C) Consuming the GalT binding site in purified ZP3 by pregalactosylation with GalT and UDPGal destroys its sperm-binding activity. Control assays performed n parallel included heat-inactivated GalT. The level of sperm binding to 2-cell embryos is indicated by the dotted line. (D)Similarly, removing the GalT binding site in ZP3 by GlcNAc’dase pretreatment destroys its sperm-binding activity, whereas pretreatment with α- or β–galactosidase has little effect (adapted from Miller et al., 1992).