Figure 1.
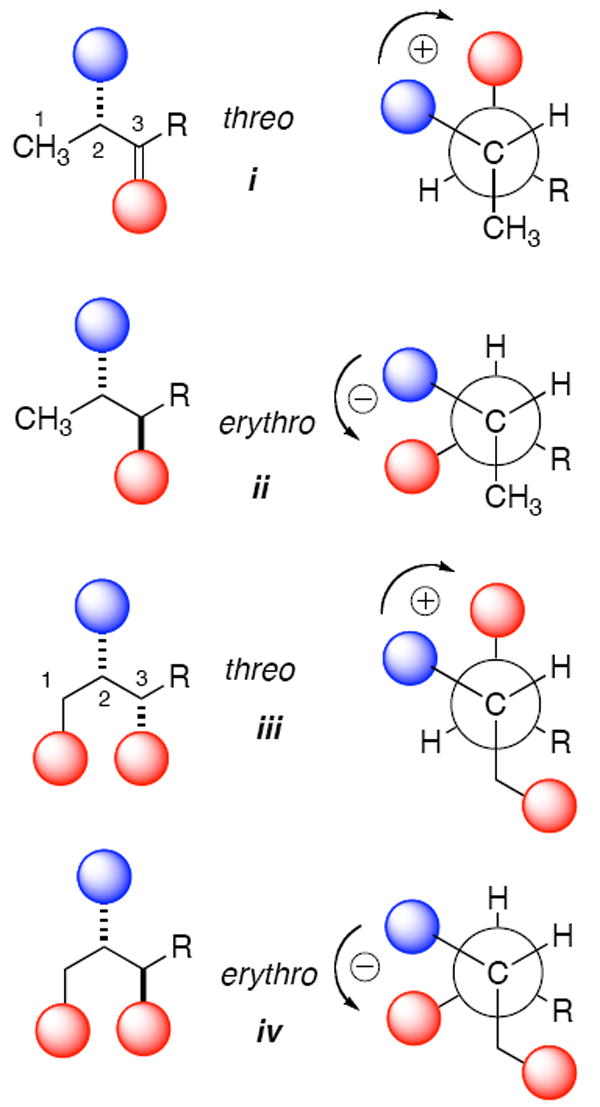
Representations of major conformers contributing to exciton coupling CD11a in threo and erythro isomers of N,O-arenecarboxy derivatives of 2-amino-3-alkanols (i and ii) and 2-amino-1,3-alkanediols (iii and iv). Blue shaded spheres represent N-arenecarboxamide chromophores and red spheres are O-arenecarboxylate chromophores (e.g. arenecarboxy = Ph(CO)O, benzoate, λmax ~228 nm). A positive exciton coupling (+ sign) is defined by a split Cotton effect with a maximum CD signal at longer wavelengths and minimum at shorter wavelengths. Contributions to ECCD from the primary C-1 chromophore (e.g. benzoate) in compounds iii and iv modulate the fine structure and intensity of the split Cotton effect but the signs remain the same as their respective counterparts i and ii. The enantiomers of i-iv would show mirror image CD spectra.
