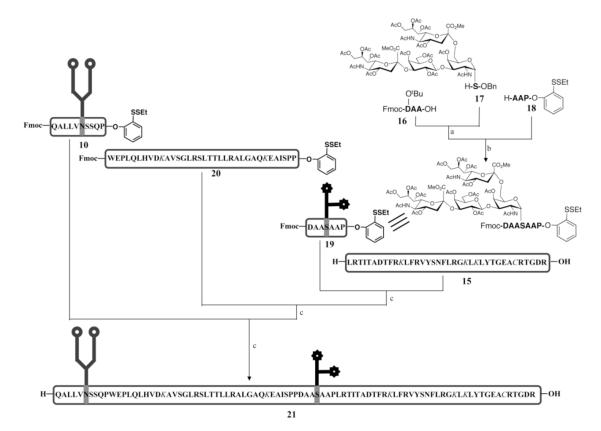
Figure 5.
Synthesis of the EPO(78-166) glycopeptide with both N-linked glycan and O-linked protected glycans.a
a The protected amino acid residues are shown in italic. Reagents and conditions: (a) DCC, DMAP, CH2Cl2, 70%; (b) 1. Pd/C, H2, MeOH, AcOH, 2. DCC, DMAP, CH2Cl2, 3. TFA, PhOH, TIPS, H2O, 25%; (c) 1. TCEP•HCl, HOOBt, DIEA, DMSO, 2. Piperidine, DMSO, 43% for the first condensation; 53% for the second condensation; 39% for the third condensation. The side chain of aspartic acid residue in peptide 16 is protected with tert-butyl group. The side chains of lysine and cysteine residues in 15, 20 and 21 are protected with ivDde and Acm, respectively.