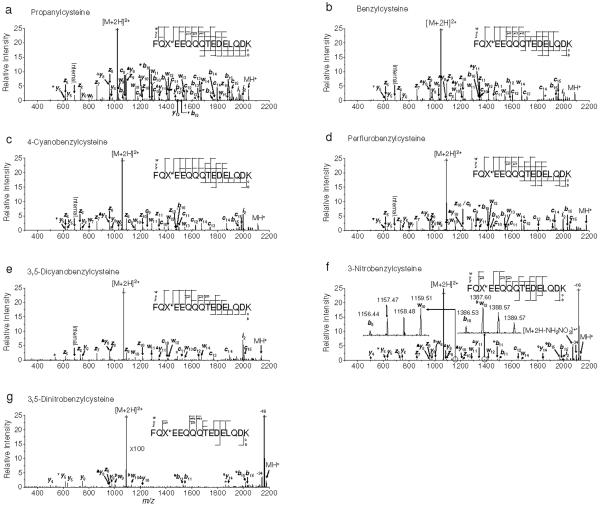
Figure 3.
IRMPD/ECD of doubly protonated model peptides. a) propanylcysteine, b) benzylcysteine, c) 4-cyanobenzylcysteine, d) perfluorobenzylcysteine, e) 3,5-dicyanobenzylcysteine, f) 3-nitrobenzylcysteine and g) 3,5-dinitrobenzylcysteine containing peptides, respectively. Precursor ions were heated by infrared photons to just below the onset of backbone cleavage. Electron irradiation was applied simultaneously with infrared excitation without isolation of heated precursor ions. Symbolic superscript appendixes °, Δ, ▼ and # indicates loss of hydroxyl radical from [b+1]+• and [y+1]+• ions, and ammonia, water and ethylene from either b and y or w ions, respectively. An asterisk indicates instrumental noise.