Table 2.
Optimization of the Ligand/Palladium Combination for the Cross Coupling of K+9- with 16a.a
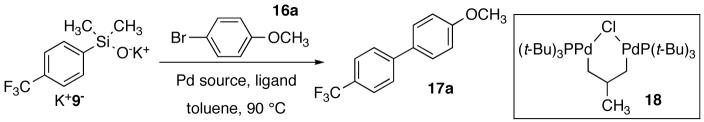 | |||||
|---|---|---|---|---|---|
| entrya | Pd source, 5 mol% Pd |
ligand, 1/1 Pd/L |
time, h |
conversion, %b |
product yield, %c |
| 1 | Pd(dba)2 | Ph3P(O) | 7 | 24 | 7 |
| 2 | (C3H5)CpPd | dppp | 20 | 100 | 30 |
| 3 | PdCl(C3H5)(Ii-Pr) | - | 20 | 43 | 10 |
| 4 | (Ph3P)4Pd | - | 20 | 68 | 37 |
| 5 | [allylPdCl]2 | - | 7 | 37 | 9 |
| 6 | [allylPdCl]2 | dppp(O)2 | 7 | 95 | 45 |
| 7 | [allylPdCl]2 | dppp(O) | 7 | 54 | 30 |
| 8 | [allylPdCl]2 | dppp | 3 | 69 | 51 |
| 9 | [allylPdCl]2 | Ph3As | 7 | 22 | 12 |
| 10 | [allylPdCl]2 | SPhos | 7 | 71 | 46 |
| 11 | [allylPdCl]2 | t-Bu3P | 5.5 | 99 (100)d | 79 (89)d |
| 12 | [allylPdCl]2 | t-Bu3P•HBF4 | 20 | 88 | 68 |
| 13 | 18 | - | 7 | 90 | 73 |
| 14 | (t-Bu3P)2Pd (19) | - | 5 | 100 (100)e | 88 (92)e |
Reactions conditions: 1.5 equiv of arylsilanolate K+9- and 1.0 equiv of 16a
Conversion was based on consumption of aryl bromide as determined by GC analysis using an internal standard.
Yield determined by GC analysis using an internal standard.
Yield in parentheses based on 2:1 ratio of ligand/Pd.
Conversion and yield in parentheses refers to the use of (t-Bu3P)2Pd purchasedfrom Aldrich Chemical Co.
