Table 9.
Cross Coupling of Electron-Deficient Potassium Arylsilanolates with Aryl Bromides Using (t-Bu3P)2Pd.a
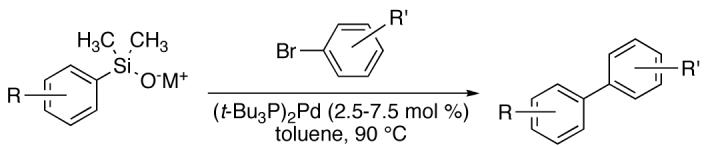 | ||||||||
|---|---|---|---|---|---|---|---|---|
| entry | R | no. | aryl halide | no. | catalyst loading. mol % |
time, h |
product | yield, % |
| 1 | 4-CO2t-Bu | K+15- | 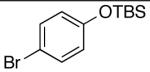 |
16n | 2.5 | 6 | 28n | 57d |
| 2 | 4-CO2t-Bu | K+15- | 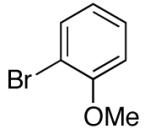 |
16j | 2.5 | 7 | 28j | 69 d |
| 3 | 4-CO2t-Bu | K+15- | 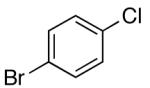 |
16h | 2.5 | 6 | 28h | 79 d |
| 4 | 4-[2-(1,3- dioxolane)] |
K+7- | 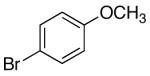 |
16a | 5.0 | 3 | 29a | 77 c |
| 5 | 4-[2-(1,3- dioxolane)] |
K+7- | 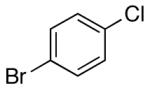 |
16h | 5.0 | 3 | 29h | 77 d |
| 6 | 4-Cl | K+10- | 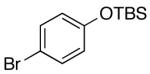 |
16n | 2.5 | 3.5 | 30n | 57 d |
| 7 | 4-Cl | K+10- | 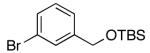 |
16r | 5 | 3.5 | 30r | 60 d |
| 8 | 4-Cl | K+10- | 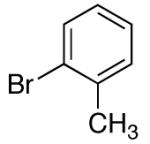 |
16c | 5 | 3.5 | 30c | 68 c |
| 9 | 4-Cl | K+10- | 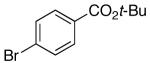 |
16l | 2.5 | 3.5 | 30l | 42 d |
| 10 | 4-Cl | K+10- | 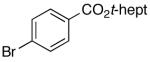 |
16s | 2.5 | 3.5 | 30s | 56 d |
| 11 | 4-Cl | K+10- | 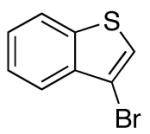 |
16q | 5.0 | 3.5 | 30q | 61 c |
| 12 | 2,6-Clb | Na+14- | 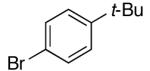 |
16g | 7.5 | 3 | 31g | 68 e |
| 13 | 2,6-Clb | Na+14- | 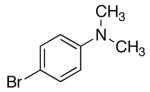 |
16k | 7.5 | 3 | 31k | 76 d |
| 14 | 2,6-Clb | Na+14- | 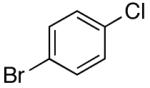 |
16h | 7.5 | 3 | 31h | 70 e |
Reactions employed 1.5 equiv of arylsilanolate
Reaction employed 2.0 equiv of arylsilanolate in THF
Yields are for isolated, chromatographically homogeneous products.
Yields are for analytically pure products.
Products were determined to be >99% pure by 1H NMR spectroscopy.
