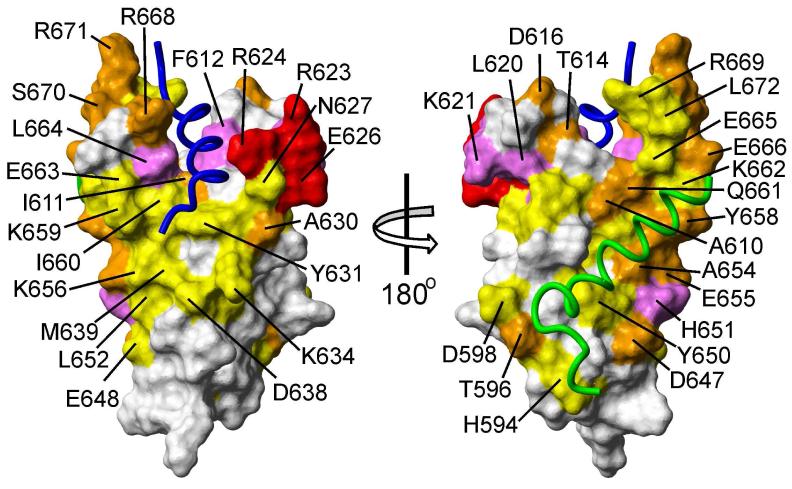
Figure 5.
Binding sites of p53 TAD on KIX. Weighted average chemical shift differences Δδ(N,H)av of KIX amide resonances (Fig. 4a) between free KIX and in complex with p53(13-61) are mapped onto the surface of KIX in the ternary KIX:MLL:c-myb complex (64) (PDB 2AGH), using colors to indicate changes in chemical shift greater than 2 × standard deviation from the mean (SD) (red), between 1 and 2 × SD (magenta), between mean and 1 × SD (orange), and between half of the average chemical shift difference ( Δδav/2) and the average chemical shift difference ( Δδav) (yellow). The polypeptide backbones of the bound MLL and c-Myb are shown in blue and green, respectively. The figure was prepared using MOLMOL (65).