Table 2.
Conversion of alkenes to pinacolboronates 11 using 1
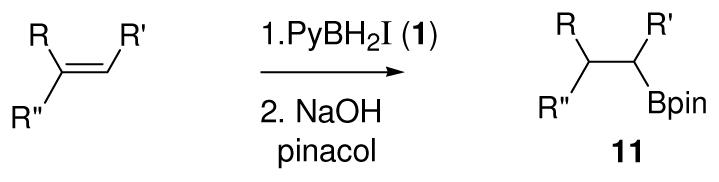 | ||||
|---|---|---|---|---|
| Entry | R, R’ | R” | Equiv 1 (PyBH2I) |
Yield(a),(b) |
| 1 | nC8H17, H | H | 2 | 40% |
| 2 | nC10H21, H | H | 2 | 40% |
| 3 | Ph, H | H | 2 | 50% |
| 4 | BzO(CH2)4, H | H | 2 | 26% |
| 5 | BzO(CH2)4, H | H | 6 | 55% |
| 6 | norbornene | H | 2 | 47% |
| 7 | norbornene | H | 6 | 68% |
| 8 | -C4H8- | H | 1.5 | 67% |
| 9 | -C4H8- | Ph | 2 | 67%(c) |
HB in DCM at RT, 2 h, followed by stirring with pinacol and NaOH (2-fold and 3-fold excess, respectively, relative to 1), 15 h, unless noted; isolated yields.
The pinacol boronates from entries 1-3 and 6-9 have been described previously and were identified by NMR comparisons; see Supporting Information for references.
HB required 15 h to go to completion.
