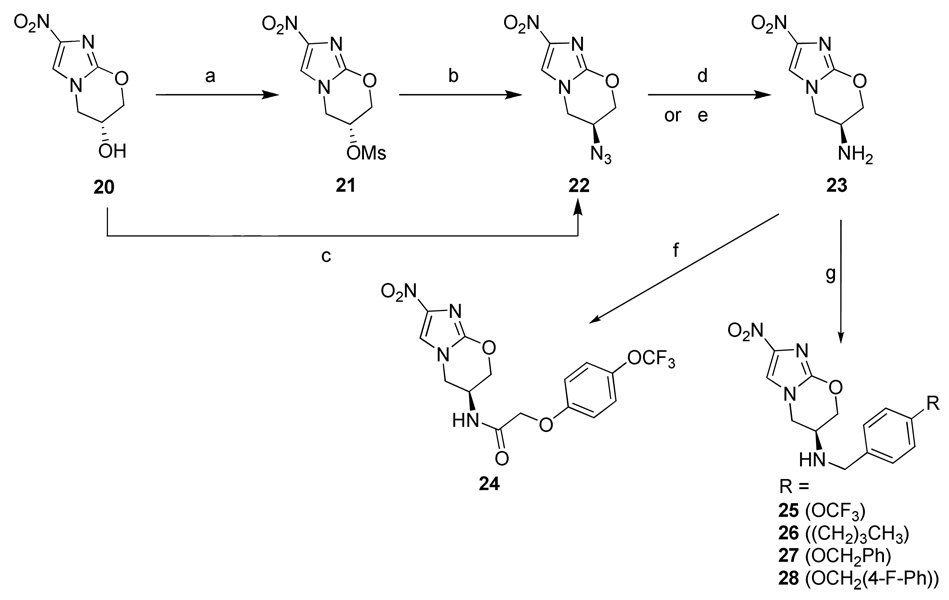
Scheme 3.
Synthesis of compounds 24–28.a
aReagents and conditions: (a) MsCl, Et3N, DMF, 0°C, 1 h; (b) NaN3, DMF, 70°C, 2 h; (c) DTAD, PPh3, (PhO)2P(=O)N3, THF, 0°C to rt, 18 h; (d) 10% Pd/C, H2 (g), EtOAc, rt, 2 h; (e) SH(CH2)3SH, Et3N, rt, 5 min; (f) 4-trifluoromethoxyphenoxyacetyl chloride, Et3N, rt, 18 h; (g) aldehyde, AcOH, NaBH3CN, DMF, rt, 2 h.