Table 1.
Bicyclic and Tricylic Diazenes
| Entry | Ketone | Diazene | Yield (%)a |
|---|---|---|---|
| 1 |
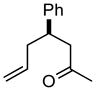 1a |
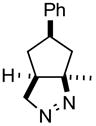 2ab |
84 |
| 2 |
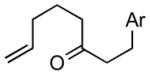 1bc |
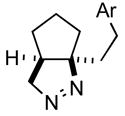 2b |
76 |
| 3 |
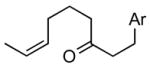 1cd |
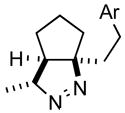 2ce |
70 |
| 4 |
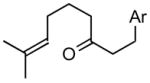 1d |
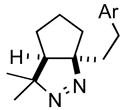 2d |
68 |
| 5 |
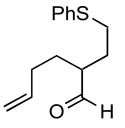 1e |
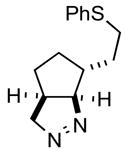 2ef |
91 |
| 6 |
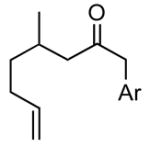 1f |
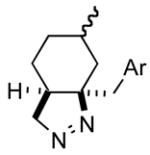 1fg |
72 |
| 7 |
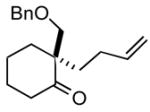 1g |
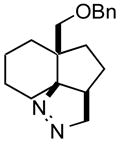 2g |
68 |
Yields are for pure isolated products.
The product was a ~ 5:1 mixture of diastereomers.
Ar = 4-methoxyphenyl.
The alkene was a ~ 5: 1 Z/E mixture.
The product was a ~ 5:1 mixture of diastereomers.
The product was a ~ 6:1 mixture of diastereomers. The major product had a 13C NMR methine at δ = 100.2. The minor diastereomer 13C NMR methine was at δ = 95.1
The product was a ~ 1:1 mixture of diastereomers.
