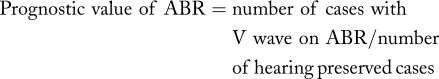ABSTRACT
We developed a cochlear nerve action potential (CNAP) monitoring technique using a microdissector and compared the results of CNAP and auditory brainstem response (ABR) monitoring. Thirty-six patients underwent vestibular schwannoma resection via the retrosigmoid approach to preserve hearing. Both CNAP with the microdissector and surface ABR were recorded during the operation. We used the microdissector as an intracranial electrode for CNAP monitoring. The CNAP waveform was classified into four types: triphasic, biphasic, positive, and flat. At the completion of the tumor resection, the triphasic waveform was observed in 11 patients and the biphasic waveform was observed in 11 patients. Hearing function was preserved in all of them, although it was preserved in only two patients with other CNAP waveform types. The prognostic value of CNAP is significantly higher than that of ABR. We found that although CNAP with a microdissector does not provide real-time monitoring, with the classification of waveforms it can be used as predictable tool for postoperative hearing more accurately than ABR.
Keywords: cochlear nerve action potential, microdissector, auditory brainstem response, vestibular schwannoma
Hearing preservation in vestibular schwannoma surgery has become increasingly common because small tumors can be found by magnetic resonance imaging (MRI).1 Neurophysiological intraoperative monitoring is important for the preservation of hearing in vestibular schwannoma surgery.2 Auditory brainstem response (ABR) and cochlear nerve action potential (CNAP) are common techniques for intraoperative monitoring.3,4 Auditory brainstem response explores the far-field responses from the cochlear nerve to the ascending auditory pathways. These responses are less dependent on neural synchrony than those recorded in the near field. Another problem with ABR involves electrical interference, which results in an increase in artifact response.5 Cochlear nerve action potential is directly recorded in more rapid recording time and has less averaging when compared with ABR recording. Therefore, CNAP is less affected by electrical artifact or desynchronous firing.6,7 However, the presence of an electrode within the surgical field can be problematic.8,9 We used the microdissector (which is made of stainless steel and insulated by Teflon), excluding the functional edge, as an intracranial electrode for CNAP monitoring. We intermittently recorded CNAP with this microdissector. We could confirm the status of the cochlear nerve as well as identifying the nerve, when it was necessary, for example, before removal of tumor, during dissection from the nerve, or at the completion of the tumor resection. Here, we report on a CNAP monitoring technique using a microdissector and compare the results of CNAP and ABR monitoring.
CLINICAL MATERIAL AND METHOD
From November 2003 to May 2007, 36 patients at our institution underwent vestibular schwannoma resection via the retrosigmoid approach to preserve hearing. Tumor was preoperatively and postoperatively evaluated by MRI. Tumor size was calculated based on intra- and extrameatal tumor extension. The tumor extension classification system developed in Hannover, Germany10 was applied: class T1, intrameatal tumor; class T2, intra-extrameatal tumor; class T3a, lesion filling the cerebellopontine cistern; class T3b, tumor reaching the brainstem; class T4a, lesion compressing the brainstem; and class T4b, tumor severely dislocating the brainstem and compressing the fourth ventricle. Pure tone average and speech discrimination testing were performed preoperatively at 1 to 7 days before surgery and at ~2 weeks after surgery. Hearing was graded according to the new Hannover Classification.11 Hearing classes H1 to H3, corresponding to a pure tone average of up to 60 dB and speech discrimination score of more than 40%, were defined as functional.12
Monitoring Procedure
Both CNAP with the microdissector and surface ABR were recorded with the Nicolet Viking Selection Evoked Potential Unit (VIASYS, Dublin, OH). Surface ABR was recorded using a subdermal needle electrode in the ipsilateral earlobe and noninverting forehead (Fpz) electrode. Cochlear nerve action potential was recorded with the microdissector (Fig. 1). This microdissector touches to the structure of interest at the operative field after the dura matter is opened and CNAP is recoded. The stimuli used both by ABR and CNAP are clicks presented at a rate of 19.7 Hz and at the amplitude of 100 dB. During surface ABR recording, 2000 repetitions are required to produce an interpretable waveform. For CNAP recording, 100 repetitions are initially required. For the identification of the cochlear nerve, we usually needed to record CNAP at several portions. The waveform at different places is compared, the part with a large waveform remains, and the removal goes forward. The tumor can be removed using the same microdissector used for CNAP recording. We finally distinguish the nerve from the tumor by considering the CNAP recording and microscopic findings. In these cases, after the confirmation of the cochlear nerve, CNAP was recorded with 25 to 50 repetitions. We were always able to confirm the status of the cochlear nerve by CNAP with the microdissector. The average acquisition time for intraoperative ABR and CNAP was, respectively, 3 to 4 minutes and 2 to 5 seconds. The waveform of CNAP was classified in four types: triphasic, biphasic, positive and flat (Fig. 2). We usually recorded CNAP when the ABR waveform changed and at the completion of the tumor resection. Electromyography (EMG) recordings of the orbicularis oris and oculi muscles by NIM-response (Medtronic, Minneapolis, MN) were used to monitor facial nerve function via a pair of subdermal needle electrodes. Monopolar stimulation with intensity of 0.1–3.0 mA was used to assess facial nerve response using the same microdissector that was used for the CNAP recording. The CNAP recording and the response on EMG with the microdissector are used to identify the nerve. The microdissector has various shapes (ball, round, ring curette, knife, and so on), and which to choose in a given situation is determined by usage (Fig. 1, right).
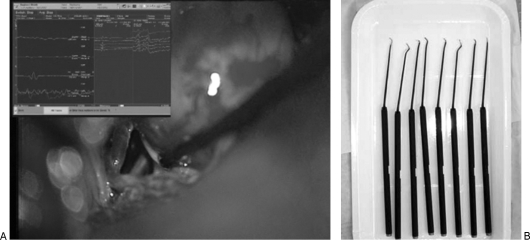
Figure 1.
(A) Microscopic intraoperative photograph (left) shows a CNAP recording with the microdissector. (B) The various shapes of the microdissector are in the photograph on the right.
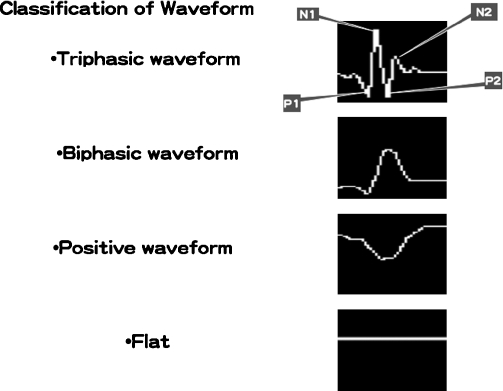
Figure 2.
Classification of waveform. N1, first negative peak; N2, second negative peak; P1, first positive peak; P2, second positive peak.
Prognostic Value
At completion of the tumor resection, we recorded both ABR and CNAP. The waveform type of CNAP at the most proximal portion of the cochlear nerve was analyzed. We estimated whether the presence of wave V on ABR or CNAP could predict postoperative hearing more accurately. The prognostic values of CNAP and ABR were calculated as follows:
Data Analysis
The results were compared and analyzed using the McNemar test. Statistical significance was defined as a probability value <0.05.
RESULT
Patient Data
The average age of the 36 patients was 48.9 years old (range, 22 to 67 years). Twenty of the patients were women and 16 were men. The tumor extension classification system revealed 1 patient with class T1, 14 patients with class T2, 11 patients with class T3a, 6 patients with class T3b, and 4 patients with class T4a. The hearing classification system revealed 10 patients in H1, 14 patients in H2, and 12 patients in H3 (Table 1).
Table 1.
Tumor Extension and Preoperative Hearing Level According to the New Hannover Classification
| H1 | H2 | H3 | |
|---|---|---|---|
| T1 | 1 | 0 | 0 |
| T2 | 5 | 6 | 3 |
| T3a | 3 | 4 | 4 |
| T3b | 0 | 3 | 3 |
| T4a | 1 | 1 | 2 |
Classification of Waveform Type and Postoperative Hearing
Cochlear nerve action potential recording typically revealed a triphasic waveform before tumor resection. It was observed that the response had a small positive deflection followed by a larger negative deflection. A second positive deflection could be discerned (Fig. 2). During surgery this triphasic waveform underwent various changes. At the completion of the tumor resection, the triphasic waveform was observed in 11 patients and the biphasic waveform was observed in 11 patients. Postoperative mean central nervous system of the patients with triphasic waveform was 28.1 dB (range 10 to 40). Three cases were Class H1 and 8 cases were Class H2. Postoperative mean pure-tone average (PTA) of the patients with biphasic waveform was 41.9 dB (range, 6.3 to 57.5 dB). One case was class H1, three cases were class H2 and seven cases were class H3. At the completion of the tumor resection, positive waveform was observed in four patients. One of them lost hearing postoperatively. One case was class H3 and two cases were class H4. At the completion of the tumor resection, CNAP could not be recorded in 10 cases. Nine of them lost their hearing postoperatively and only one case was class H3 (Table 2).
Table 2.
Classification of Waveform Type at Completion of Tumor Resection and Postoperative Hearing (Mean)
| Waveform Type | Number of Patients | PTA (dB) |
|---|---|---|
| PTA, pure-tone average. | ||
| Triphasic | 11 | 28.1 |
| Biphasic | 11 | 41.9 |
| Positive | 4 | 62.9* deaf 1 case |
| Flat | 10 | 48.8* deaf 9 cases |
Mean except deaf case.
Intraoperative Auditory Assessment
Wave V on ABR was observed in 32 cases before removal of tumor. Although wave V could not be observed in four cases before removal of tumor, CNAP could be recorded with the microdissector. For these cases, we recorded CNAP frequently and tried to prevent injury to the cochlear nerve. During surgery, wave V on ABR disappeared in 15 cases. In these cases, we immediately analyzed the injured portion of the nerve. We could guess the injured portion of the nerve by the CNAP comparison of waveform type between the peripheral and proximal portions of the nerve (Fig. 3). Despite that both wave V and CNAP could not be observed at the end of surgery, hearing was preserved in one case. Wave V was observed at the end of surgery in 17 cases. Hearing was preserved in all of them. The triphasic or biphasic waveform of CNAP could be recorded in 22 cases at the completion of the tumor resection. Hearing was preserved in all of them. That is, wave V was not recorded in 5 cases.
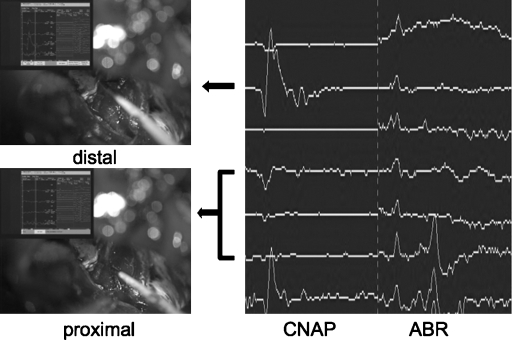
Figure 3.
A typical conduction block of the cochlear nerve. During the tumor dissection from the cochlear nerve wave V disappeared (right). The CNAP at the peripheral portion is a triphasic waveform (upper left), and that at the proximal portion is a positive waveform (lower left). This finding indicates that the place where the waveform changed was injured by manipulation. CNAP, cochlear nerve action potential; ABR, auditory brainstem response.
The prognostic value of CNAP was 91.7%, and it significantly differed from the prognostic value of ABR (70.8%).
DISCUSSION
ABR versus CNAP
Intraoperative monitoring techniques have contributed to the preservation of cranial nerve function. Cochlear nerve action potential monitoring brought a higher chance of hearing preservation.2 The CNAP monitoring technique, first reported by Møller and Jannetta,13 uses direct placement of recording electrodes close to the cochlear nerves. Although far-field ABR signal averaging typically necessitates thousands of averaged sweeps to achieve an adequate signal-to-noise ratio, CNAP has the advantage of being a near-field technique. Larger amplitude signals are observed and fewer averaged sweeps are required to obtain satisfactory waveforms. We initially averaged 100 sweeps to obtain a CNAP signal and 25 to 50 sweeps after the confirmation of the cochlear nerve. For intraoperative auditory assessment, the frequency of sweeps sometimes changed as a result of ABR or intraoperative conditions. However, for CNAP recording we averaged up to 100 sweeps at the maximum, and the longest time required was 5 seconds. Usually only 2 to 3 seconds were required. When an ABR signal clearly changed during the operation, for example, when waves I through V interpeak latency increased, or the amplitude of wave V decreased, we recorded CNAP. Then, only several seconds were required to confirm the status of the cochlear nerve by CNAP with the microdissector.
In four cases, distinct ABR signals could not be obtained from the start of operation. In these cases, we frequently recorded CNAP to confirm the state of the cochlear nerve, and only CNAP could provide the vital information. Absent waveforms on ABR, which have not been a reliable negative prognostic sign regarding hearing preservation,14 indicate either extensive nerve invasion by tumor without the potential for hearing preservation or nerve compression and neurapraxia with reasonable expectation for hearing preservation. In the latter case, CNAP monitoring is absolutely necessary because ABR cannot monitor the status of the cochlear nerve during surgery. Normalization of ABR recording in selected cases after surgery has been reported.15
Cochlear nerve action potential monitoring is not particularly widespread, although it can provide near real-time monitoring and important information, because the intracranial electrode sometimes obstructs the surgical manipulation.8,9 We did not place electrodes at any time during the operation. Therefore, CNAP with the microdissector cannot provide close real-time information; however, important information can be obtained, although it takes an extra few seconds.
CNAP Parameters
For CNAP, the latency of the first negative peak (N1) and amplitude ratio after the first positive peak (P1) are usually measured.16 Because the place of measurement is not fixed and the shape of the electrodes (microdissector) in this technique vary, absolute amplitude and latency are not reliable. Therefore, we evaluate the morphology of CNAP. We classified the waveforms of CNAP into four types, based on the experimental result by Sekiya et al.17 Sequential changes of evoked action potentials (EAPs) from the internal auditory meatus (IAM) portion of the cochlear nerve were recorded during cerebellopontine angle manipulations in dogs. In that experiment, the earliest change was a depression of the P2-N2 complex, progressing to complete obliteration of the P2-N2 complex. These are respectively named the P2-N2 obliterating stage and the P1-N1 stage. Next, N1 disappeared and it becomes the P1 stage. In the P2-N2 obliterating stage, the wave II, III, and IV amplitude decreased with prolongation of the wave I to IV interpeak latency, and wave IV was sometimes barely discernible in brainstem auditory evoked potentials. Only wave I was discernible in the P1 stage. We modified this classification and simply classified it into four types. The difference between the biphasic waveform and the positive waveform is in whether N1 is found. Because all cases with triphasic or biphasic waveform preserved their hearing, the presence of N1 of CNAP must be a good index for postoperative hearing.
Identification of Cochlear Nerve
In operations for large tumors, we could not identify the nerve at the early stage of tumor resection. First, we performed internal decompression at the portion without a CNAP signal. Then, we checked CNAP at several portions again. If a CNAP was observed, we dissected and removed portions other than those where the CNAP signal was present. Thus, we could guess the location of the cochlear nerve by considering the amplitude of CNAP and the microscopic findings. Identification of the cochlear nerve by bipolar recording is superior to this technique. Bipolar recording can clearly indicate the cleavage plane between the tumor and the cochlear nerve.18 Because the technique we presented is a monopolar recording, it was necessary to compare the measurement results in different places. We need to guess the location of the cochlear nerve by considering the amplitude of CNAP and the microscopic findings. However, it is possible to remove the portion safely without a response. With this technique, we can dissect the tumor easily without exchanging the tool, because the same microdissector can be used for both tumor dissection and CNAP measuring. Even if the bipolar recording probe is used, it is necessary to record frequently to identify the nerve. The merit of this technique is that the tool does not have to be exchanged frequently.
Feedback for Surgical Manipulation
Cochlear nerve action potential monitoring can also detect the damaged portion of the nerve during surgery. Figure 3 shows a typical conduction block of the cochlear nerve. Cochlear nerve action potential at the peripheral portion is a triphasic waveform, and at the proximal portion it is a positive waveform. This finding indicates that the place where the waveform changed was injured by manipulation. A conduction block was simulated as a phenomenon in which a depolarization wavefront stops traveling when it reaches a certain point, although the following repolarization wavefront continues to travel until it reaches the same point.19 Thus, CNAP monitoring could indicate the injured portion of the nerve during surgery. The same findings have been reported previously,20,21 where a silver electrode wire with a small cotton wick was used; it was placed on the proximal portion of the eighth nerve. The authors moved it from the proximal to the distal portion of the nerve to the conduction velocity and the site of the lesion at the end of the surgery. We think that the handling of the microdissector is easier than that of the silver electrode wire with a small cotton wick. At any rate, CNAP monitoring is considered useful for providing surgeons with important feedback during surgical manipulation.
CONCLUSION
Although CNAP with a microdissector does not provide real-time monitoring, it can predict postoperative hearing more accurately than ABR. We quickly and simply identified the cochlear nerve using this technique. Therefore, this technique is very useful for hearing preservation in vestibular schwannoma surgery.
REFERENCES
- Tucker A, Slattery W H, III, Solcyk L, Brackmann D E. Intraoperative auditory assessments as predictors of hearing preservation after vestibular schwannoma surgery. J Am Acad Audiol. 2001;12(9):471–477. [PubMed] [Google Scholar]
- Abramson M, Stein B M, Pedley T A, Emerson R G, Wazen J J. Intraoperative BAER monitoring and hearing preservation in the treatment of acoustic neuromas. Laryngoscope. 1985;95(11):1318–1322. doi: 10.1288/00005537-198511000-00004. [DOI] [PubMed] [Google Scholar]
- Danner C, Mastrodimos B, Cueva R A. A comparison of direct eighth nerve monitoring and auditory brainstem response in hearing preservation surgery for vestibular schwannoma. Otol Neurotol. 2004;25(5):826–832. doi: 10.1097/00129492-200409000-00029. [DOI] [PubMed] [Google Scholar]
- Schmerber S, Lavieille J P, Dumas G, Herve T. Intraoperative auditory monitoring in vestibular schwannoma surgery: new trends. Acta Otolaryngol. 2004;124(1):53–61. doi: 10.1080/00016480310014840. [DOI] [PubMed] [Google Scholar]
- Jackson L E, Roberson J B., Jr Acoustic neuroma surgery: use of cochlear nerve action potential monitoring for hearing preservation. Am J Otol. 2000;21(2):249–259. doi: 10.1016/s0196-0709(00)80018-6. [DOI] [PubMed] [Google Scholar]
- Nedzelski J M, Chiong C M, Cashman M Z, Stanton S G, Rowed D W. Hearing preservation in acoustic neuroma surgery: value of monitoring cochlear nerve action potentials. Otolaryngol Head Neck Surg. 1994;111(6):703–709. doi: 10.1177/019459989411100602. [DOI] [PubMed] [Google Scholar]
- Rowed D W, Nedzelski J M, Cashman M Z, Stanton S, Harrison R V. Cochlear nerve monitoring during cerebellopontine angle operations. Can J Neurol Sci. 1988;15(1):68–72. doi: 10.1017/s0317167100027220. [DOI] [PubMed] [Google Scholar]
- Linden R D, Tator C H, Benedict C, Charles D, Mraz V, Bell I. Electrophysiological monitoring during acoustic neuroma and other posterior fossa surgery. Can J Neurol Sci. 1988;15(1):73–81. doi: 10.1017/s0317167100027232. [DOI] [PubMed] [Google Scholar]
- Zappia J J, Wiet R J, O'Connor C A, Martone L. Intraoperative auditory monitoring in acoustic neuroma surgery. Otolaryngol Head Neck Surg. 1996 l;115(1):98–106. doi: 10.1016/S0194-5998(96)70144-4. [DOI] [PubMed] [Google Scholar]
- Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery. 1997;40(1):11–21. doi: 10.1097/00006123-199701000-00002. [DOI] [PubMed] [Google Scholar]
- Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): the facial nerve–preservation and restitution of function. Neurosurgery. 1997;40(4):684–694. doi: 10.1097/00006123-199704000-00006. [DOI] [PubMed] [Google Scholar]
- Samii M, Gerganov V, Samii A. Improved preservation of hearing and facial nerve function in vestibular schwannoma surgery via the retrosigmoid approach in a series of 200 patients. J Neurosurg. 2006;105(4):527–535. doi: 10.3171/jns.2006.105.4.527. [DOI] [PubMed] [Google Scholar]
- Møller A R, Jannetta P J. Compound action potentials recorded intracranially from the auditory nerve in man. Exp Neurol. 1981;74(3):862–874. doi: 10.1016/0014-4886(81)90259-4. [DOI] [PubMed] [Google Scholar]
- Roberson J B, Jr, Jackson L E, McAuley J R. Acoustic neuroma surgery: absent auditory brainstem response does not contraindicate attempted hearing preservation. Laryngoscope. 1999;109(6):904–910. doi: 10.1097/00005537-199906000-00012. [DOI] [PubMed] [Google Scholar]
- Hoehmann D. Pre- and postoperative hearing thresholds and brainstem responses in patients with acoustic neuroma: follow-up study using the middle fossa approach. Am J Otol. 1991;12(3):172–178. [PubMed] [Google Scholar]
- Colletti V, Fiorino F G, Mocella S, Policante Z. ECochG, CNAP and ABR monitoring during vestibular schwannoma surgery. Audiology. 1998;37(1):27–37. doi: 10.3109/00206099809072959. [DOI] [PubMed] [Google Scholar]
- Sekiya T, Iwabuchi T, Kamata S, Ishida T. Deterioration of auditory evoked potentials during cerebellopontine angle manipulations. An interpretation based on an experimental model in dogs. J Neurosurg. 1985;63(4):598–607. doi: 10.3171/jns.1985.63.4.0598. [DOI] [PubMed] [Google Scholar]
- Rosenberg S I, Martin W H, Pratt H, Schwegler J W, Silverstein H. Bipolar cochlear nerve recording technique: a preliminary report. Am J Otol. 1993;14(4):362–368. [PubMed] [Google Scholar]
- Tani T, Ushida T, Yamamoto H, Okuhara Y. Waveform changes due to conduction block and their underlying mechanism in spinal somatosensory evoked potential: a computer simulation. Technical note. J Neurosurg. 1997;86(2):303–310. doi: 10.3171/jns.1997.86.2.0303. [DOI] [PubMed] [Google Scholar]
- Colletti V, Fiorino F G. Advances in monitoring of seventh and eighth cranial nerve function during posterior fossa surgery. Am J Otol. 1998;19(4):503–512. [PubMed] [Google Scholar]
- Colletti V, Bricolo A, Fiorino F G, Bruni L. Changes in directly recorded cochlear nerve compound action potentials during acoustic tumor surgery. Skull Base Surg. 1994;4(1):1–9. doi: 10.1055/s-2008-1058981. [DOI] [PMC free article] [PubMed] [Google Scholar]