Figure 2.
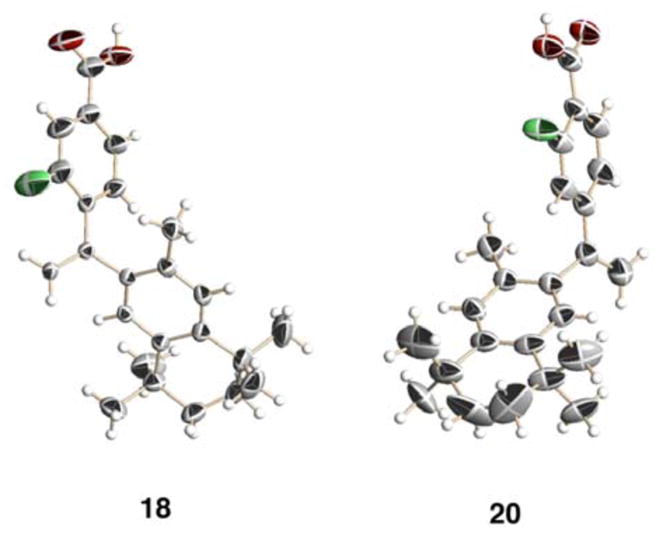
The X-ray crystal structures of fluorinated bexarotene analogs 18 and 20. Compound 18 crystallized in such a way that a channel whose boundaries were formed by the carboxylic acid groups of 18 incorporated a solvent that could not be identified by the model, though likely candidates such as ethyl acetate (the crystallization solvent) were examined. Compound 20 displayed twist-isomerism in the aliphatic ring, as well as partial-filled occupancies of the fluorine atom at both ortho-positions to the carboxylic acid group due to carbon-carbon single bond rotation. Additionally, the carbonyl carbon-oxygen bond of the carboxylic acid group of 20 could not be clearly resolved. Hence, one twist isomer, as well a given rotational isomer for the fluorine and carboxylic acid groups, has been displayed.
