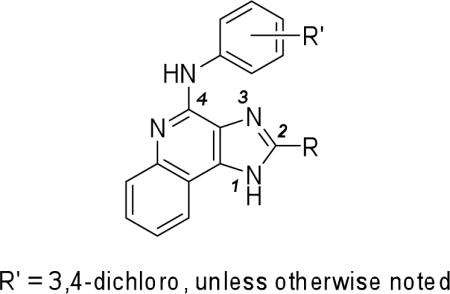
Table 2.
Potency of 2-cycloalkyl substituted 1H-imidazo-[4,5-c]quinolin-4-amine derivatives in binding assays at the human A1 and A3ARs expressed in CHO cells and at the human A2A in HEK-293 cells and the allosteric effects at the human A3AR.a
 | ||||||
|---|---|---|---|---|---|---|
| No. | R = | A1AR, %displ. at 10 μM | A2AAR, %displ. at 10 μM | A3AR, %displ. at 10 μM | A3AR ag. dissociation,c % at 10 μM | [35S]GTPγS binding in A3AR cells,d % at 10 μM |
| 1b | 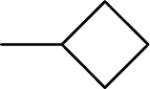 |
−5.2 | 0.4 | 52 | 116±3 | 126±3b |
| 2b | 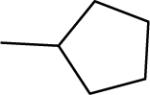 |
15 | 0 | 67e | 144±9 | 141±5b |
| 3 | 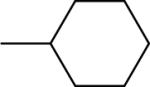 |
37±9 | −11.2±2.8 | 40.5±13.4 | 192±7 | 208±14 |
| 4b | 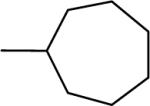 |
−4.2 | −2.2 | 68 | 130±2 | 115±7b |
| 16 | 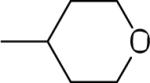 |
37.2±8.6 | −11.9±2.8 | 69.6±1.4 | 115±12 | 114±7 |
| 17 | 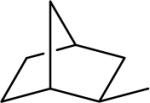 |
77.9±12.2 | 5.8±1.0 | 61.2±1.4 | 187±16 | 201±26 |
| 18 | 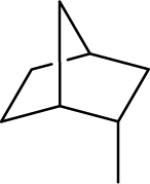 |
44.8±10.5 | 39.4±1.9 | 50.5±5.5 | 168±8 | 168±13 |
| 19 | 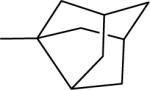 |
14.5±2.7 | −17.3±6.9 | 3.3±4.1 | 139±10 | 156±2 |
| 20 | 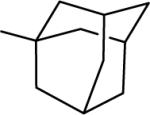 |
13.8±6.9 | −4.4±12.5 | 17.2±6.7 | 196±13 | 210±12 |
| 21 | 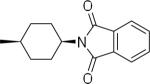 |
21.4±4.3 | 56.8±1.7 | 5.6±4.9 | 102±5 | 96±5 |
| 22 | 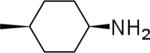 |
31.7±16.2 | 15.8±1.2 | 4.2±6.1 | 107±11 | 111±6 |
| 23 | 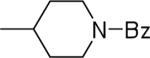 |
20.2±10.1 | 8.8±1.9 | 29.8±0.6 | 91±9 | 93±7 |
| 24 | 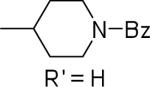 |
12.4±1.1 | 43.4±5.5 | 35.3±1.5 | 92±5 | 93±1 |
| 25 | 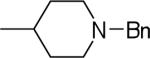 |
17.8±3.6 | −3.1±1.6 | 46.3±0.8 | 93±6 | 97±5 |
| 26 | 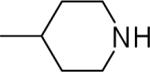 |
10.9±8.6 | 7.0±0.7 | 52.8±1.1 | 101±7 | 99±3 |
All experiments were performed using adherent mammalian cells stably transfected with cDNA encoding the human ARs. Binding at human A1, A2A and A3ARs in this study was carried out as described in the Experimental Procedures using [3H]34, [3H]35, or [125I]36 as a radioligand.19-21 Values from the present study are expressed as mean ± s.e.m., n = 3−5. Percentage inhibition at A1, A2A, or A3 receptors is expressed as the mean value from 2−4 separate experiments with similar results performed in duplicate.
Values from Göblyös et al,15 the functional enhancement of which was measured with the cyclic AMP assay.26
Dissociation: % decrease of [125I]36 dissociation at 60 min (control = 100%).
Increase of efficacy in the stimulation of the binding of [35S]GTPγS: compared to maximal effect induced by 10 μM 15 alone (set to 100%). It should be noted that the Emax of 15 in this functional assay was recently demonstrated to be about 50% of that of 37.17 In the adenylate cyclase assay, 15 and 37 were both full agonists.15
Ki value in a binding assay15 = 4690±970 nM.
