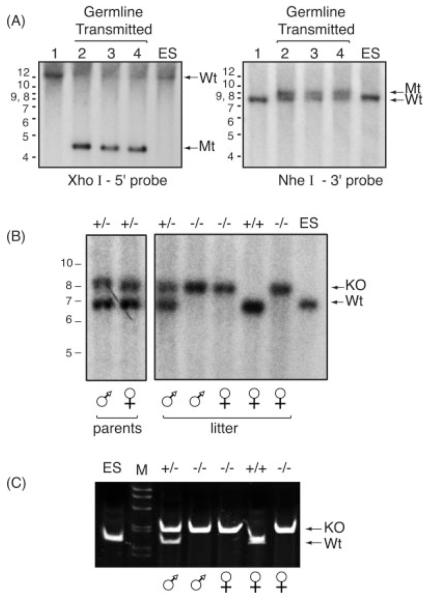
FIG. 2.
Genomic DNA analysis confirmed the presence of the Tead2 mutant allele. (A) Genomic DNA was extracted from the tails of pups born from matings between chimeric males and C57BL/6J females. DNA was digested with the indicted restriction endonuclease, subjected to Southern blotting-hybridization, and then detected with the indicated probe (Fig. 1A, B). Lanes 2–4 contain DNA from agouti pups. Lane 1 contains DNA from a black littermate. XhoI-5′ probe gives rise to a 19.9 kb DNA fragment from the wild type (Wt) allele, and a 4.6 kb DNA fragment from the mutant allele (Mt). NheI-3′ probe gives rise to 8.4 kb fragment from the Wt allele, and a 9.5 kb fragment from the mutant allele. Genomic DNA from WT ES cells was included as a positive control. (B) Mice containing the mutant allele were mated to EllaCre+ mice, and resulting pups were assayed for the deleted/KO allele. Genomic DNA was digested with EcoRV restriction endonuclease, subjected to Southern blotting-hybridization, and the DNA detected with “KO probe” (Fig. 1C). The Wt allele generates a 7.2 kb DNA fragment (Wt). The knockout allele generates a 8.5 kb DNA fragment (KO). Result shown is an example from such matings (“parents”). These Tead2 heterozygous parents (male and female) were then mated to each other and their litters of five pups were similarly assayed for the presence of KO allele. (C) The DNA samples (“litter”) in panel B were also subjected to multiplex genomic DNA PCR analysis. Primers A and B (Fig. 1B) amplified a 165 bp fragment from the Wt allele. Primers A and C (Fig. 1C) amplified a 210 bp fragment from the KO allele.