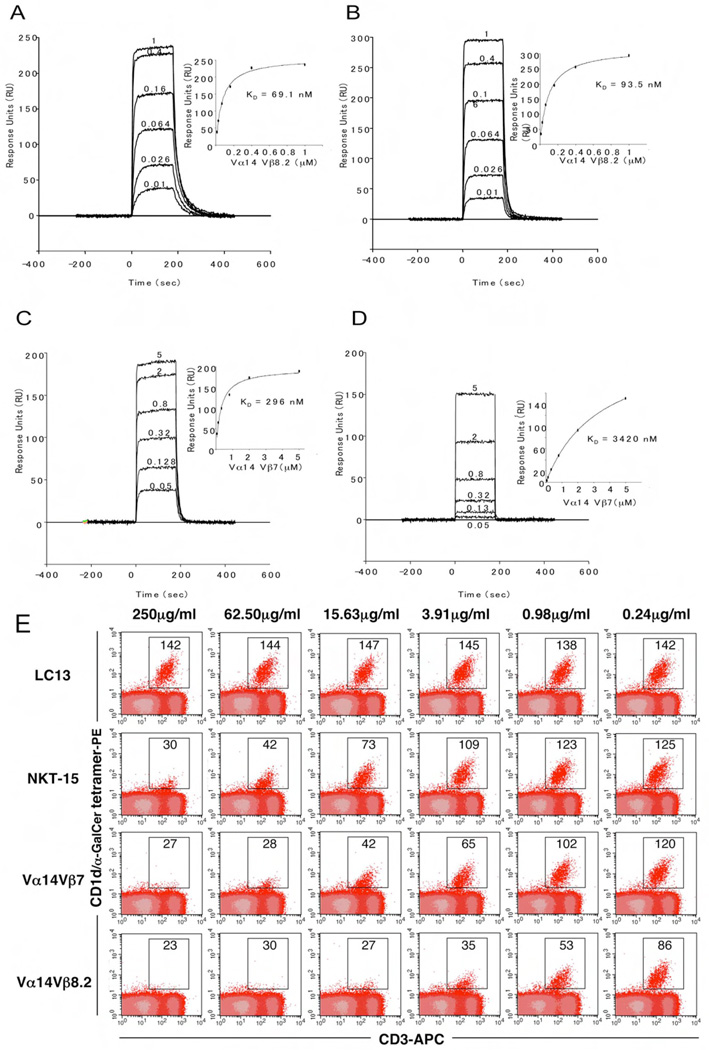
Figure 4.
Differential binding affinities of NKT TCRs to CD1d-α-GalCer. Vα14Jα18-Vβ8.2 (A and B) and Vα14Jα18-Vβ7 (C and D) NKT TCR were injected over streptavidin immobilised mouse (A and C) and human (B and D) CD1d-α–GalCer and simultaneously over a control cell coated with unloaded CD1d. Sensograms show the binding (response units, RU) of increasing concentrations of TCR (0.01 to 1µM for Vα14Jα18-Vβ8.2 and 0.05 to 5 µM for Vα14Jα18-Vβ7) to mouse and human CD1d-α–GalCer following baseline subtraction. Insets show saturation plots demonstrating equilibrium binding of NKT TCR to immobilised CD1d-α–GalCer. The equilibrium dissociation constants (KD) derived by equilibrium analysis were equivalent to those derived by kinetic analysis. E) CD1d-α–GalCer tetramer inhibition. Recombinant soluble NKT TCRs were examined for their ability to block binding of mCD1d/αGC tetramers to mouse NKT cells. PE labelled CD1d-α–GalCer tetramers were pre-incubated with titrating amounts of soluble NKT TCRs or an irrelevant TCR control, LC13, before staining of mouse thymocytes. Cells were analysed by flow cytometry showing mCD1d-α–GalCer tetramer-PE on the vertical axis and anti-CD3 APC on the horizontal axis. CD3+ mCD1d-α–GalCer tetramer+ thymic NKT cells are indicated within the square with the MFI (mean fluorescence intensity) indicated. All measurements were taken in duplicate.